
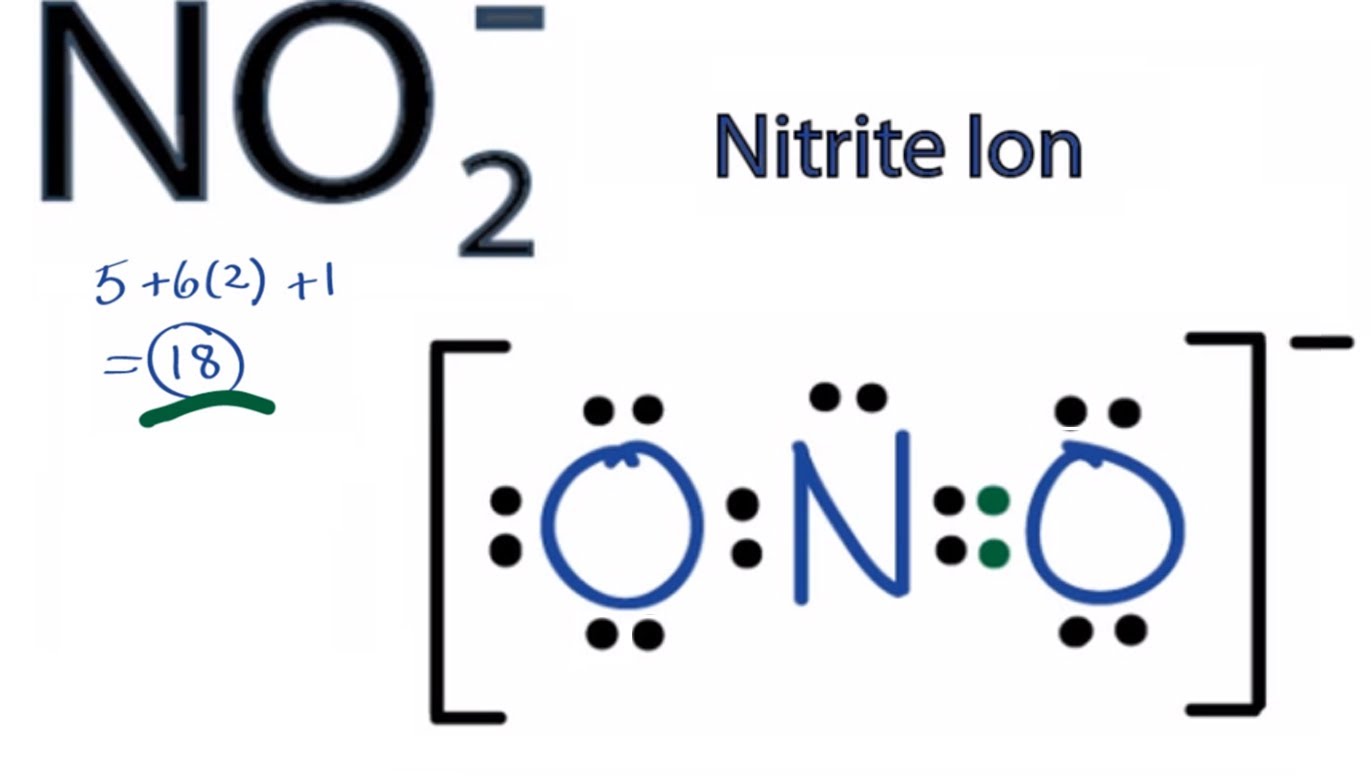
NO2 (Nitrogen Dioxide) Lewis Dot Structure Science Trends
Learn about TEAM LEWIS Munich (Germany) office. Search jobs. See reviews, salaries & interviews from TEAM LEWIS employees in Munich (Germany).
Drawing Lewis Structures Chemistry Socratic
MUNICH, October 28, 2021: Stuttgart Airport is expected to become a hub for regional electric flights with zero operating emissions, following the agreement between Stuttgart Airport and Lilium. Stuttgart is joining the planned southern German network which already consists of the Munich and Nuremberg airports, as previously announced.The Munich-based aviation company Lilium, positioned to be.

NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
The Lewis structure of N2O, also known as nitrous oxide, involves two nitrogen atoms (N) and one oxygen atom (O). Nitrogen has 5 valence electrons, while oxygen has 6 valence electrons. The total number of valence electrons in N2O is calculated as follows: 2 (N) + 1 (O) = 2 (5) + 1 (6) = 10 + 6 = 16 valence electrons.
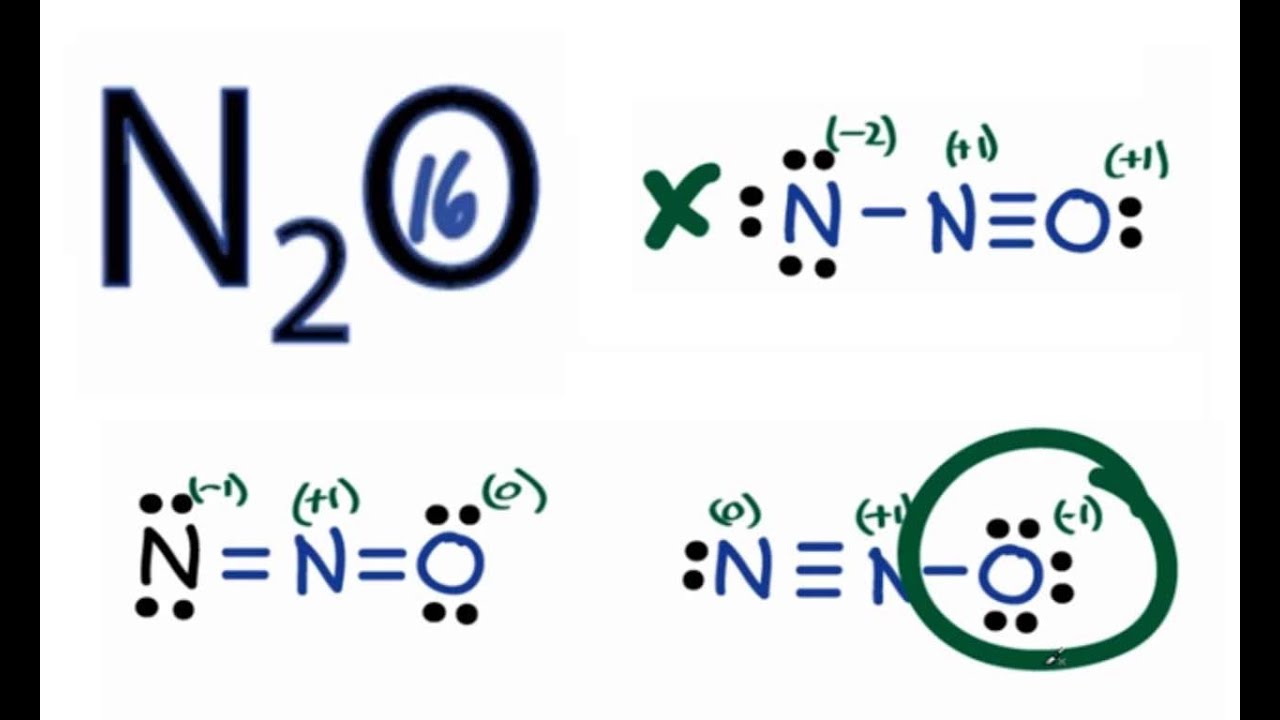
N2O Lewis Structure How to Draw the Lewis Structure for N2O YouTube
A step-by-step explanation of how to draw the N2O Lewis Dot Structure (Nitrous oxide or Dinitrogen oxide).For the N2O structure use the periodic table to fin.

Dengan menggunakan rumus titik elektron (struktur
There are several resonance structures for N2O (Nitrous oxide). We start with a valid Lewis structure and then follow these general rules. For N2O resonance.
N2o Name
Steps of drawing N2O lewis structure Step 1: Find the total valence electrons in N2O molecule. In order to find the total valence electrons in N2O molecule, first of all you should know the valence electrons present in the nitrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
For N2O, we add up the valence electrons of nitrogen (5) and the two oxygen atoms (6 each), giving us a total of 16 valence electrons. Place the atoms around the central atom: In the N2O Lewis structure, we arrange the two oxygen atoms around the central nitrogen atom. We then connect the atoms with single bonds.
Chemistry model molecule nitrogen oxide N2O scientific element formula. Integrated particles
Step #1: Calculate the total number of valence electrons. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

N2o Molecule
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
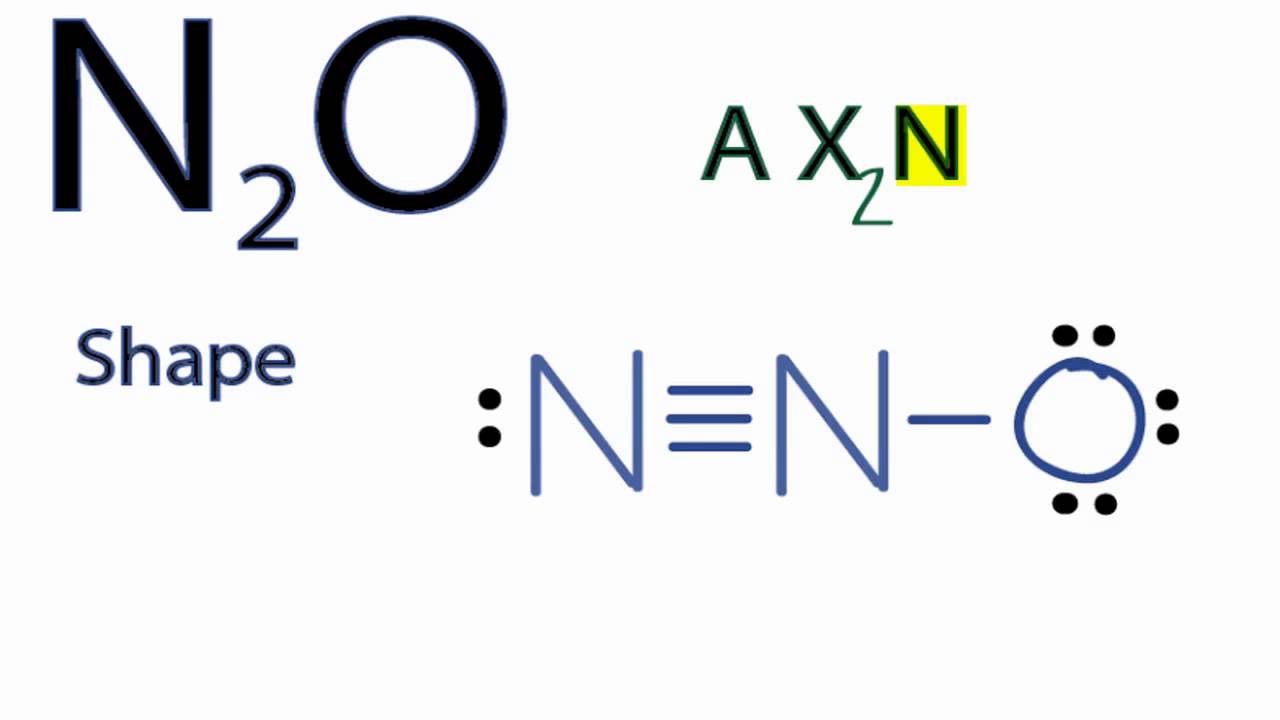
N2O Molecular Geometry / Shape and Bond Angles YouTube
A step-by-step explanation of how to draw the N2O Lewis Dot Structure (Dinitrogen monoxide or Nitrous Oxide).For the N2O structure use the periodic table to.

N2O Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
Here's how you can easily draw the N 2 O Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure.

N2O Lewis Structure Nitrous Oxide YouTube
Explanation: 16 valence electrons: 2 × 5nitrogen + 1 × 6oxygen = 8 electron pairs..to distribute over 3 centres. And you must simply KNOW here that the oxygen is terminal. And given the example, there will be formal charge separation. N ≡ + N − O− versus −N = + N = O.
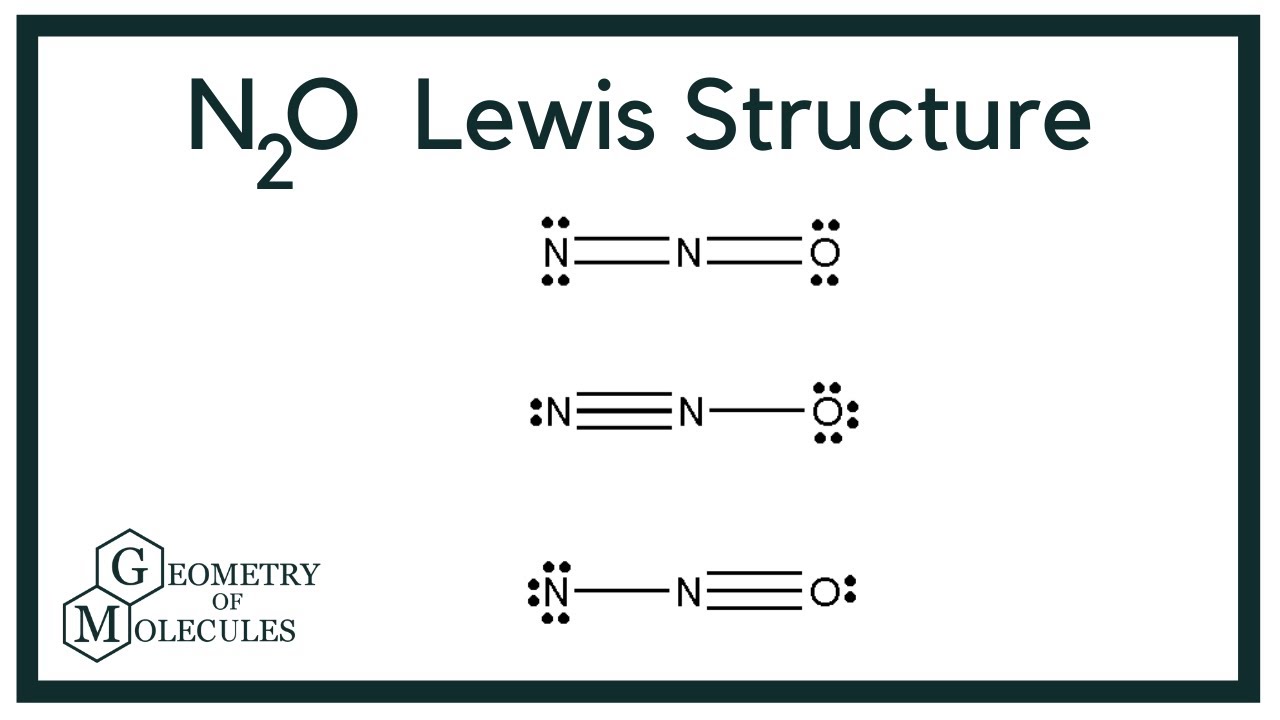
N2O Lewis Structure (Dinitrogen Oxide) YouTube
For the N 2 O Lewis structure you'll want to select the structure with formal charges closed to zero. In this case there are two similar structures. Choose the structure in which the most electronegative atom (in the case of this structure the Oxygen atom) has the negative formal charge. There are 16 valence electrons in N 2 O.

N2o nitrous oxide molecule Royalty Free Vector Image
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N2O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 1800. The.

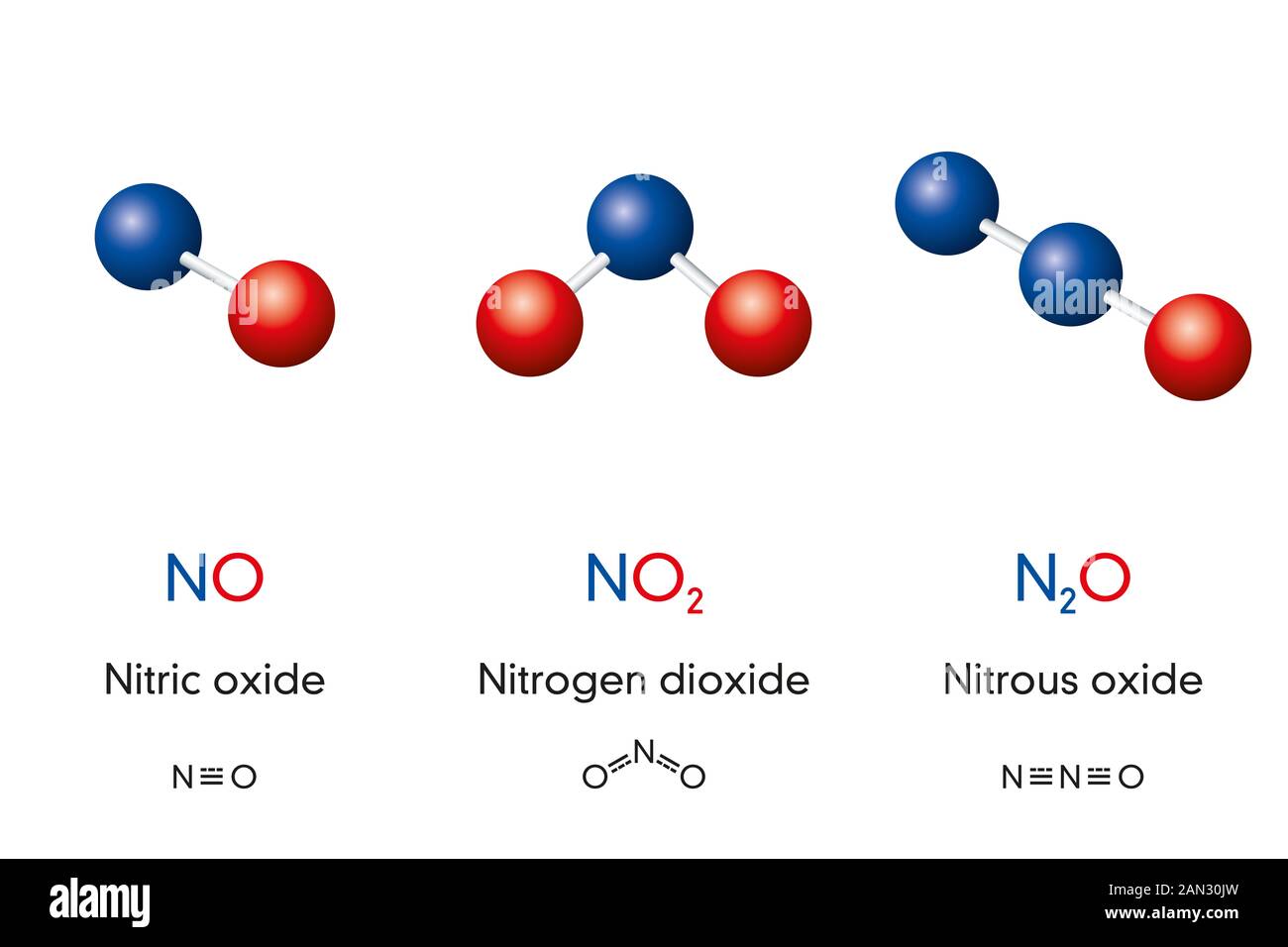
Chemical formulas and molecule model of nitrogen oxide nitric oxide NO, nitrogen dioxide NO2
Understanding the Lewis Structure of N2O. Nitrous Oxide (N 2 O) is a compound commonly encountered in Chemistry and plays a significant role in Mathematics education. This section provides a detailed explanation of the Lewis structure of N 2 O and its importance in understanding molecular geometry.. The Role of Electron Pair Repulsion Theory

QUIMICA Estructura de Lewis de N2O y carga formal AULAEXPRESS YouTube
This chemistry video tutorial explains how to draw the lewis structure of N2O also known as Nitrous Oxide or Dinitrogen Monoxide. It also covers the molecul.