
Bau der DNA
I quickly take you through how to draw the Lewis Structure of H3PO4 (Phosphoric Acid). I also go over hybridization, shape and bond angles.
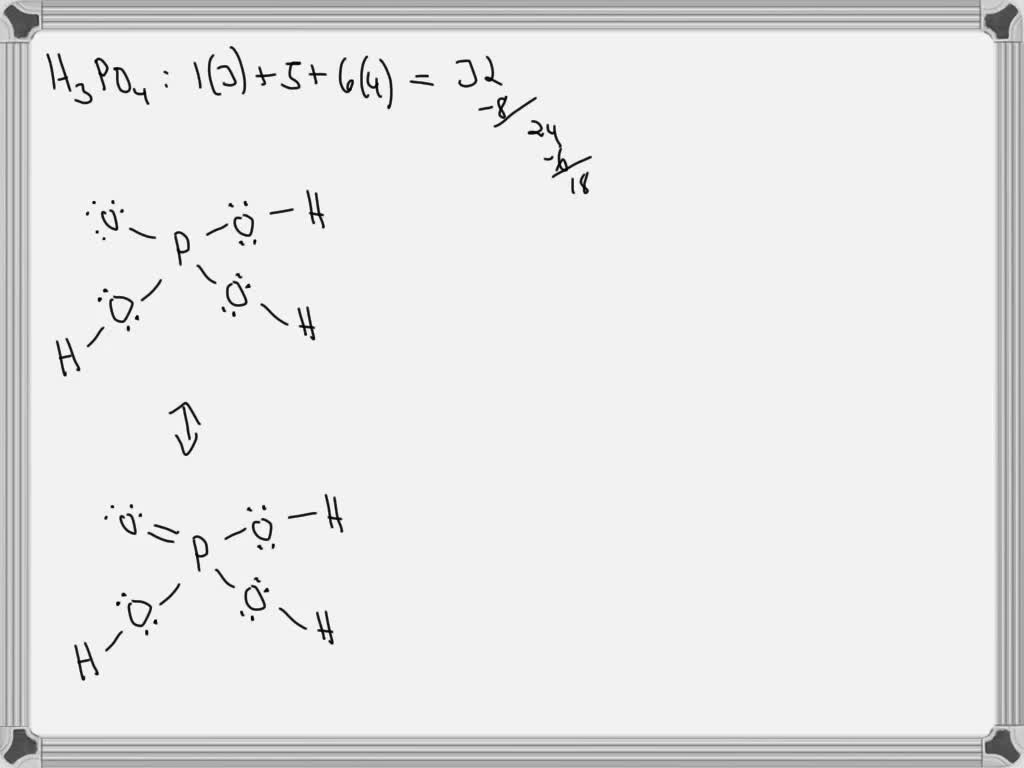
SOLVED Draw out the Lewis Structure of H3PO4, and consider its major resonance forms. Box the
August 6, 20205 min read. Phosphoric acid is a corrosive inorganic acid with the chemical formula H 3 PO 4. It's a weak acid, available in a range of quantities, purities and reagent grades. In its pure form, phosphoric acid is a colorless solid. In less concentrated form, it is odorless, viscous liquid with a density of 1.885 g/mL.

H3po4
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: A Draw a Lewis structure for H3PO4 in which the octet rule is satisfied on all atoms and show all NONZERO formal charges on all atoms. B Draw a Lewis structure for PSBr3 in which the octet rule is satisfied on.

H3PO4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
Pertanyaan. Perhatikan gambar struktur Lewis Senyawa H 3PO4 berikut! Pasangan elektron yang terbentuk secara kovalen koordinasi ditunjukkan pada nomor. (Nomor atom H = 1 ; O = 8 ; P = 15) 1. 2.

H3PO4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
In the H 3 PO 4 Lewis structure Phosphorous (P) is least electron electronegative atom and goes in the center of the Lewis structure. When we have an H (or H2 or H3) in front of a polyatomic molecule (like CO 3, PO 4, NO 2, etc.) we know that it's an acid. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
.png)
What is the Lewis Dot structure for H_3PO_4? Socratic
H 3 PO 4 (phosphoric acid) has three hydrogen atoms, one phosphorus atom, and four oxygen atoms.. In the H 3 PO 4 Lewis structure, there is one double bond and three single bonds around the phosphorus atom, with four oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the left oxygen, right oxygen, and the bottom oxygen atom (with which the hydrogen atom is.
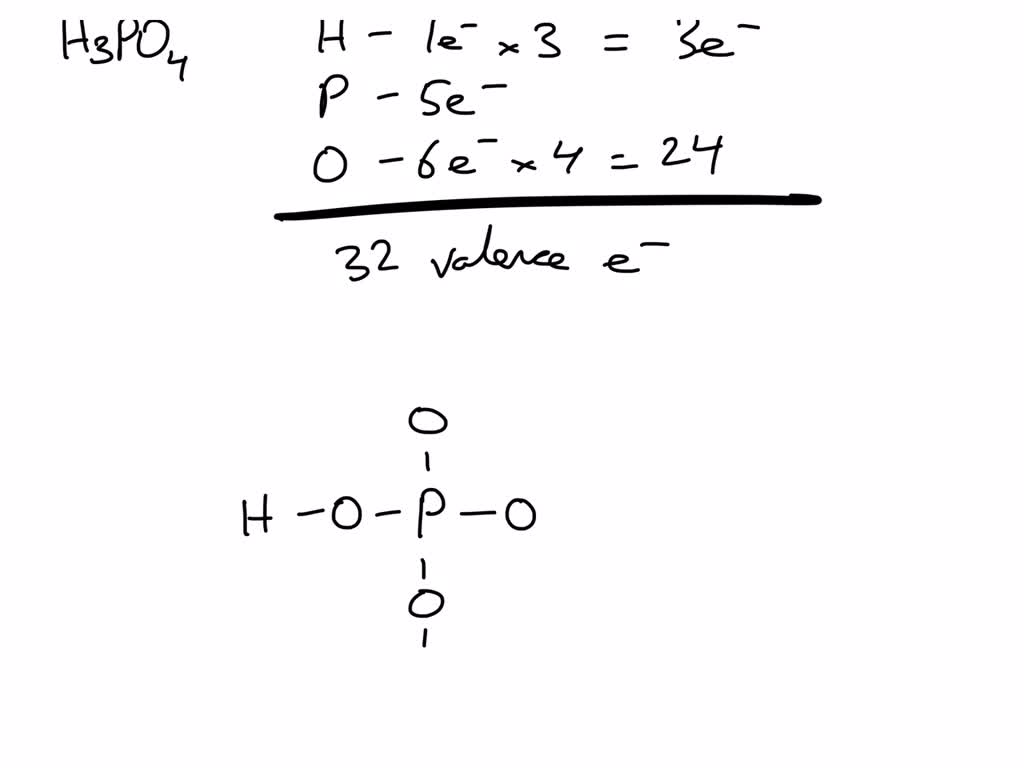
SOLVED Determine the number of valence electrons in phosphoric acid (H3PO4) and then draw the
H3PO4 Lewis Structure: How to Draw the Lewis Structure for H3PO4. Wayne Breslyn. 744. views. 01:40. HNO2 Lewis Structure: How to Draw the Lewis Structure for Nitrous Acid. Wayne Breslyn. 802. views. 02:43. HNO3 Lewis Structure - How to Draw the Lewis Structure for HNO3. Wayne Breslyn. 906. views. 05:56.
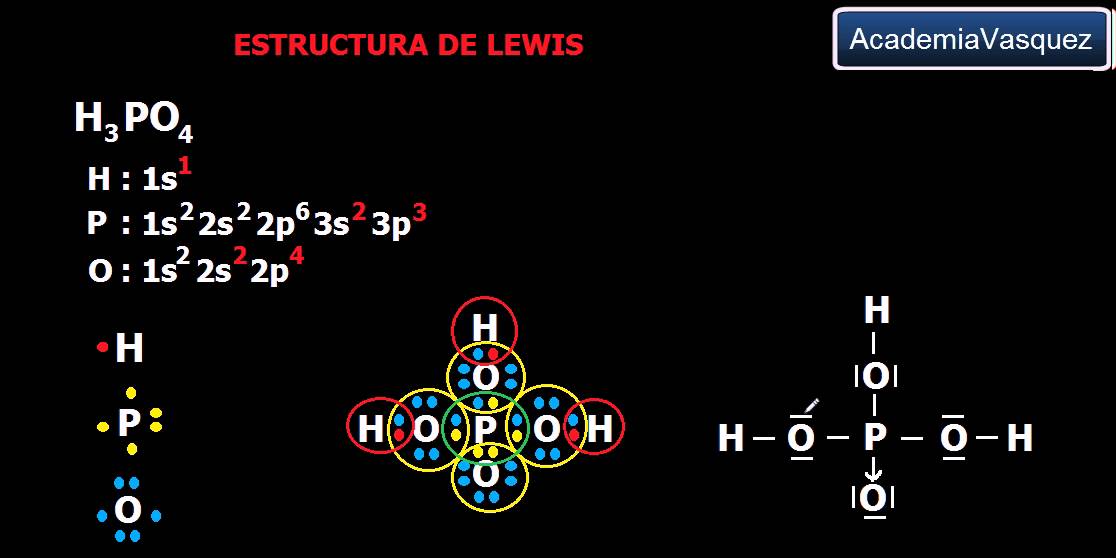
H3po4
H3PO4 is a chemical formula for Phosphoric acid and it is classified as a weak acid. This compound is also known as orthophosphoric acid. In this video we wi.
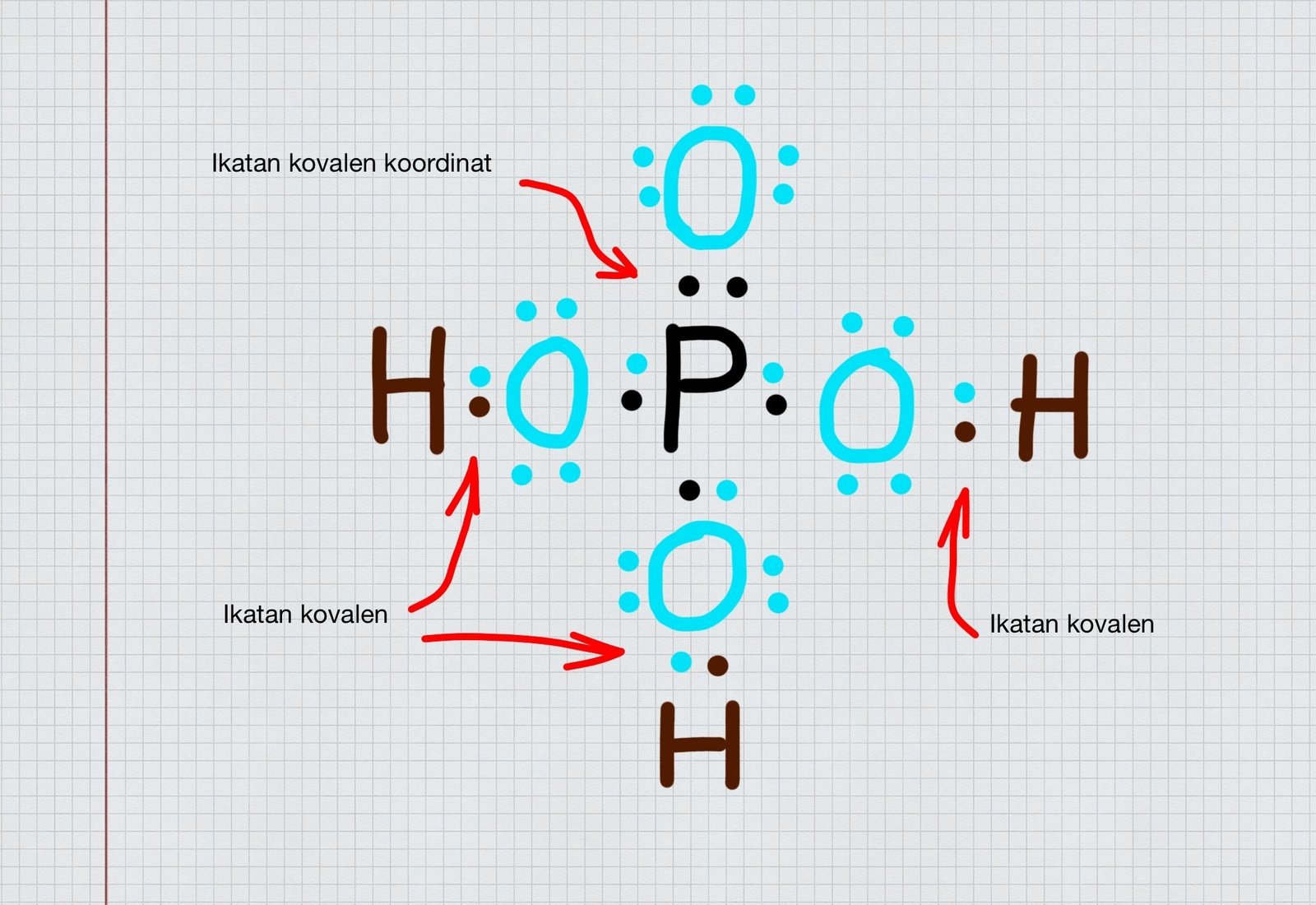
Lewis Dot Structure Of H3po4 Phosphoric Acid
Steps of drawing H3PO4 lewis structure Step 1: Find the total valence electrons in H3PO4 molecule. In order to find the total valence electrons in H3PO4 (phosphoric acid) molecule, first of all you should know the valence electrons present in hydrogen atom, phosphorus atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

H3PO4 Lewis Structure How to Draw the Lewis Structure for H3PO4 YouTube
thermodynamic physical systems. density steel alloys. scatter plot melting point vs. boiling point of metals. the pH value of milk. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics.

Phosphoric acid h3po4 Royalty Free Vector Image
Hydrogen Phosphate, H3PO4 is an important chemical compound. Phosphoric acid, also known as orthophosphoric acid is a colorless and odorless weak acid with an 85% aqueous solution. It is also available in a transparent solid-state with a density of 1.834 g/cc. Phosphoric acid has a lot of uses in daily life.

Estructura De Lewis Del Acido Fosforico Compuesto
A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure (Phosphoric acid).For the H3PO4 structure use the periodic table to find the total nu.

Lewis Structure Of H3Po4 Everything You Need To Know learnpedia.click
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

How To Draw The Lewis Structure of Phosphoric Acid (H3PO4) YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H3PO4 (phosphoric acid). In order to draw the lewis structure of H3PO4, first of all you have to find the total number of valence electrons present in the H3PO4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

rumus lewis dari H3PO4 Brainly.co.id
Steps. By using the following steps, you can easily draw the Lewis structure of H 3 PO 4. #1 Draw skeleton. #2 Show chemical bond. #3 Mark lone pairs. #4 Calculate formal charge and check stability (if octet is already completed on central atom) #5 Convert lone pair and calculate formal charge again (if formal charges are not closer to zero.

Which is the Lewis structure for H3PO4?
4. (Phosphoric Acid) Phosphoric acid (H 3 PO 4) lewis structure is drawn using the concept of total valence electrons. H 3 PO 4 contains three elements phosphorous, oxygen and hydrogen. These elements provide electrons (not all electrons) of last shell to form bonds. In this tutorial, we will learn, how to construct the lewis structure of H 3.