
How to Draw the Lewis Dot Structure for H2SO4 Sulfuric acid YouTube
A step-by-step explanation of how to draw the H2SO4 Lewis Structure (Sulfuric Acid). When we have an H (or H2) in front of a polyatomic molecule (like CO3.

Perhatikan struktur Lewis senyawa H2SO4 berikut. H O S O
Draw the Lewis structure for H2SO4, HSO4-, and SO42-. For each species, determine the maximum number of equivalent resonance structures. Sulfur is the central atom in all three species. On the other hand, if the molecule contains hydrogen atoms they are attached to the oxygen atoms. Only include the best structures, e.g. a structure with bad formal charges should not be included. The.

struktur lewis senyawa H2SO4 Brainly.co.id
H2SO4 Lewis Structure. Step 1 - in the first step, we should count the valence electrons for the H2SO4 lewis structure. In the H2SO4 lewis structure, there are three types of atoms S, O, and H present. Now S is the group 16 th element and belongs to the O family, so it has six electrons in the valence shell for S. Now O is also a group VIA element and it has also six electrons in the valence.
H2so4 Estrutura De Lewis
Here's how you can easily draw the H 2 SO 4 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure.

H2SO4 Lewis Structure Sulfuric Acid YouTube
A step-by-step explanation of how to draw the H2SO4 Lewis Dot Structure (Sulfuric acid).For the H2SO4 structure use the periodic table to find the total numb.

Struktur Lewis H2SO4 YouTube
Langkah-langkah Menggambar Struktur Lewis H2SO4 Langkah 1: Temukan jumlah total elektron valensi dalam molekul H2SO4. Untuk mengetahui jumlah total elektron valensi pada molekul H2SO4 (asam sulfat), pertama-tama Anda perlu mengetahui elektron valensi yang terdapat pada atom hidrogen, atom belerang, dan juga atom oksigen.
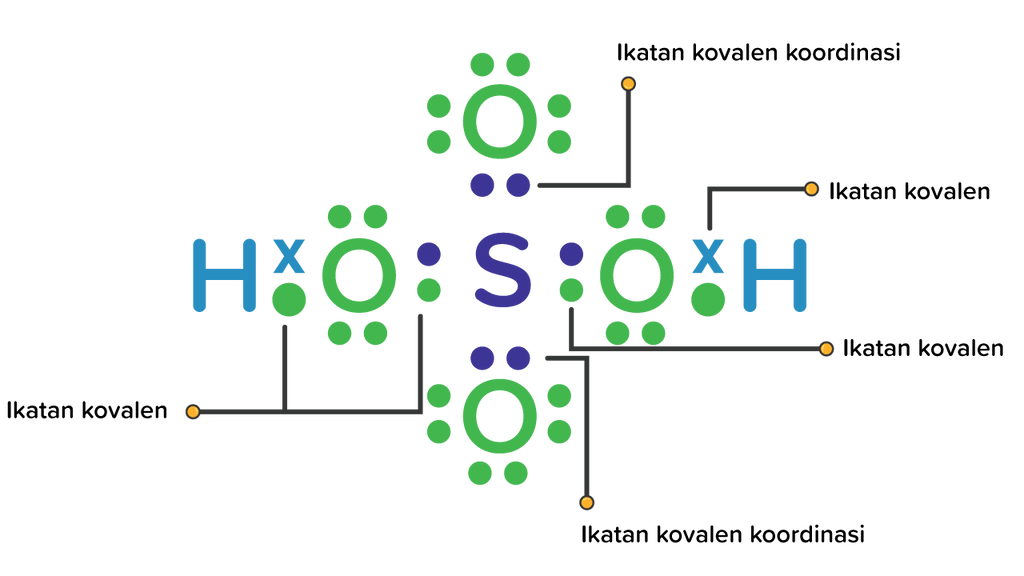
Apakah H 2 SO 4 termasuk senyawa kovalen dan dapat...
878. 05:56. H2SO4 Lewis Structure - Sulfuric Acid. The Organic Chemistry Tutor. 626. Select textbook and university. Improve your experience by picking them. 1. Intro to General Chemistry 3h 53m.

H2SO4 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
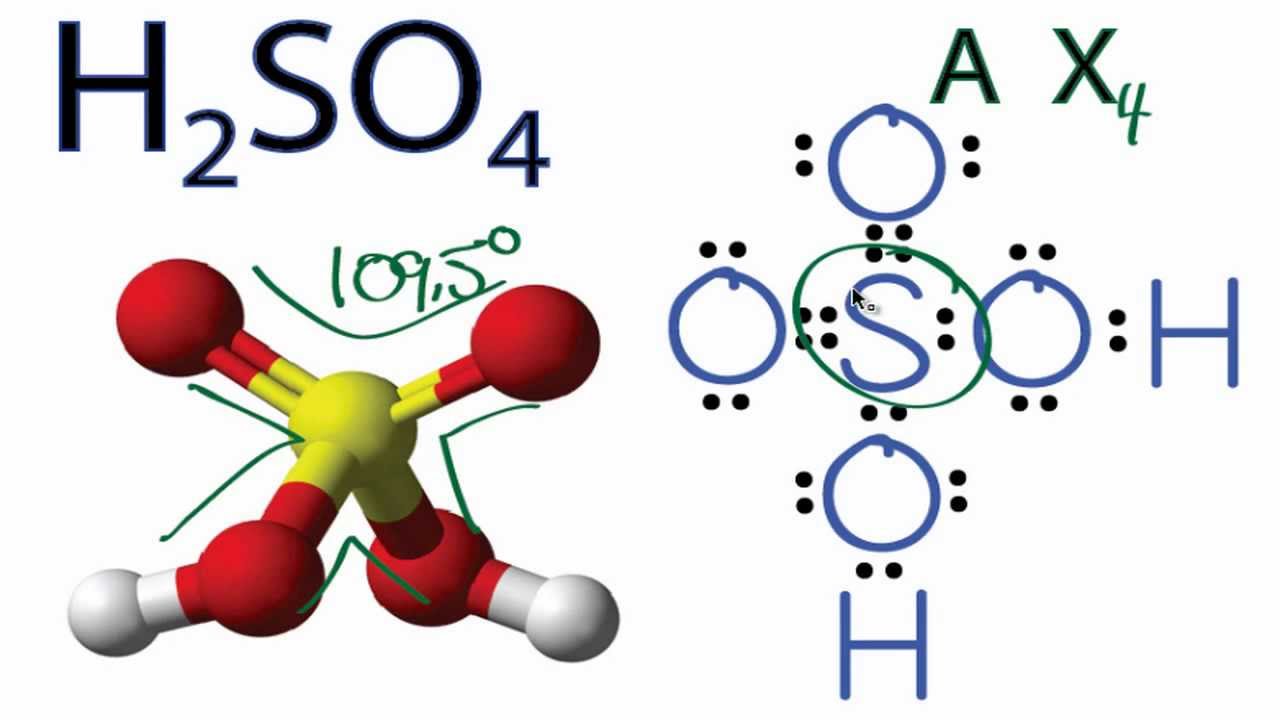
For H2SO4 we have a total of 32 valence electrons. Put a pair between the atoms. We're forming chemical bonds here. We've used 8, 10, 12, and then around the outside to fill the octets on the Oxygens, 14, and 32. So this looks like a pretty good Lewis structure for H2SO4. We've used all 32 valence electrons and each of the atoms has a full.
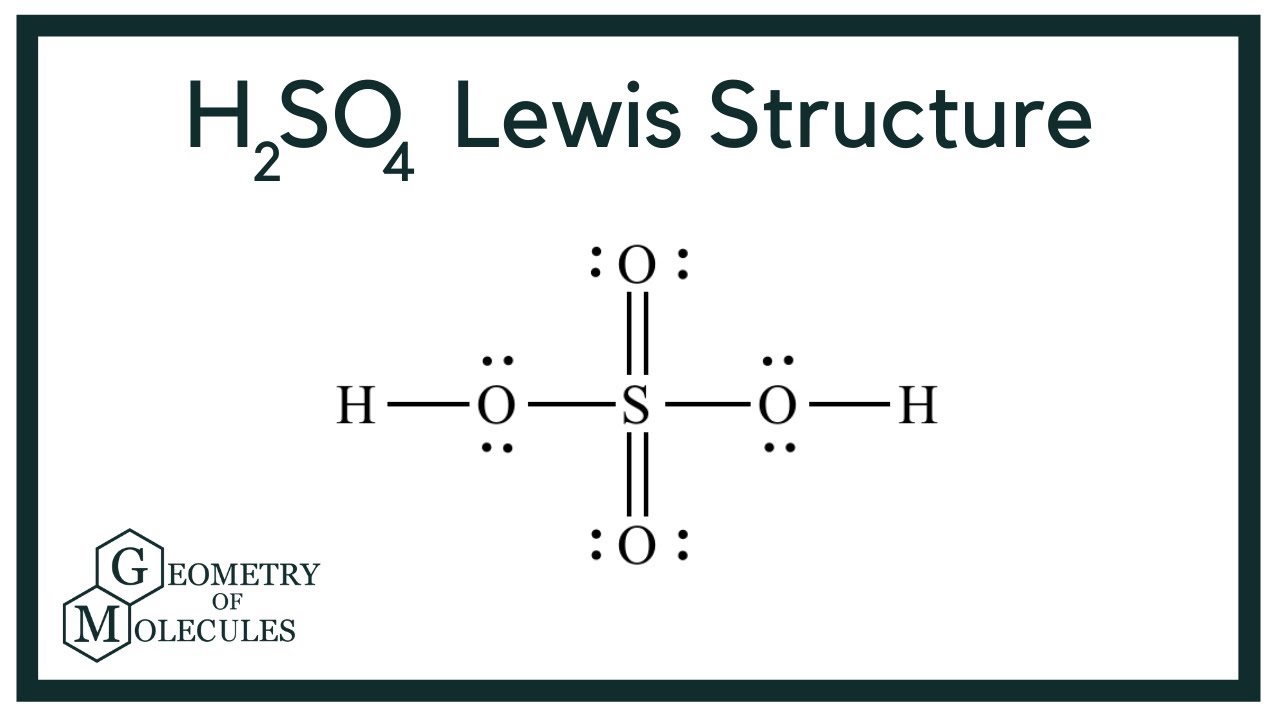
H2SO4 Lewis Structure (Sulfuric Acid) YouTube
Lewis Structure of Sulfuric Acid (H 2 SO 4) - Steps of Drawing. Lewis structure of sulfuric acid is drawn step by step in this tutorial. Total valence electrons concept is used to draw the lewis structure of H 2 SO 4. Sulfuric acid is a strong dibasic acid. Sulfuric acid | H 2 SO 4. Sulfuric acid is a dibasic strong acid.

MUATAN FORMAL3 MENENTUKAN KESTABILAN STRUKTUR LEWIS H2SO4 YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2SO4 (sulfuric acid). In order to draw the lewis structure of H2SO4, first of all you have to find the total number of valence electrons present in the H2SO4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
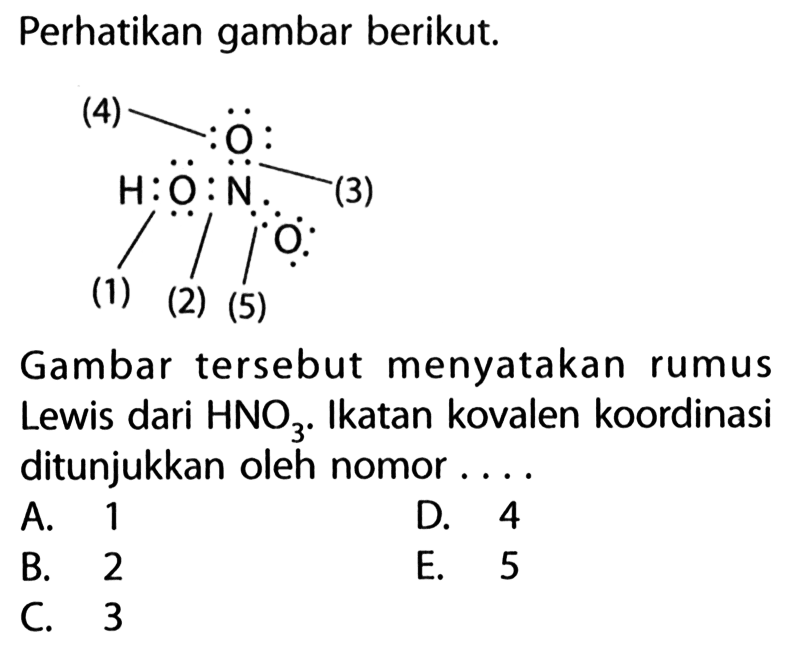
Perhatikan gambar struktur Lewis senyawa H2SO4 berikut! 3...
Steps of drawing H2SO4 lewis structure Step 1: Find the total valence electrons in H2SO4 molecule. In order to find the total valence electrons in H2SO4 (sulfuric acid) molecule, first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

QUÍMICA Estructura Lewis del H2SO4 YouTube
H2SO4 is a chemical formula of Sulfuric acid which is commonly known as Oil of Vitriol. It's a mineral acid composed of elements like oxygen, hydrogen, and sulfur. It has a molecular weight of 98.079 g/mol. H2SO4 works as an oxidizing and dehydrating agent. Furthermore, it's diprotic in nature which holds the capacity of releasing two.

Menggambarkan struktur Lewis molekul H2SO4 YouTube
I quickly take you through how to draw the Lewis Structure of H2SO4 (Sulfuric Acid). I also go over hybridization, shape and bond angles.

Perhatikan struktur Lewis H2SO4 berikut! O H O S O H O
2. Molecular geometry: The Lewis structure of H 2 SO 4 indicates that the molecule has a tetrahedral geometry with the hybridization of sp 3. 3. Polar or non-polar: The Lewis structure of H 2 SO 4 indicates that the molecule is polar because there is a difference between electronegativity of sulphur and oxygen atom.

Menggambarkan Struktur Lewis dari Senyawa H2SO4 YouTube
A panel last week indicted four of the nine teenagers accused in the beating death of 17-year-old Jonathan Lewis on November 1. They are Dontral Beaver, 16; Damien Hernandez, 18; Treavion Randolph.

Perhatikan gambar struktur Lewis senyawa H2SO4 berikut! 3...
Services for longtime Las Vegas gaming executive Leo Lewis, who casino owners and the state hired when gambling operations were in trouble, will be 1 p.m. Saturday at Palm Mortuary, 7400 W.