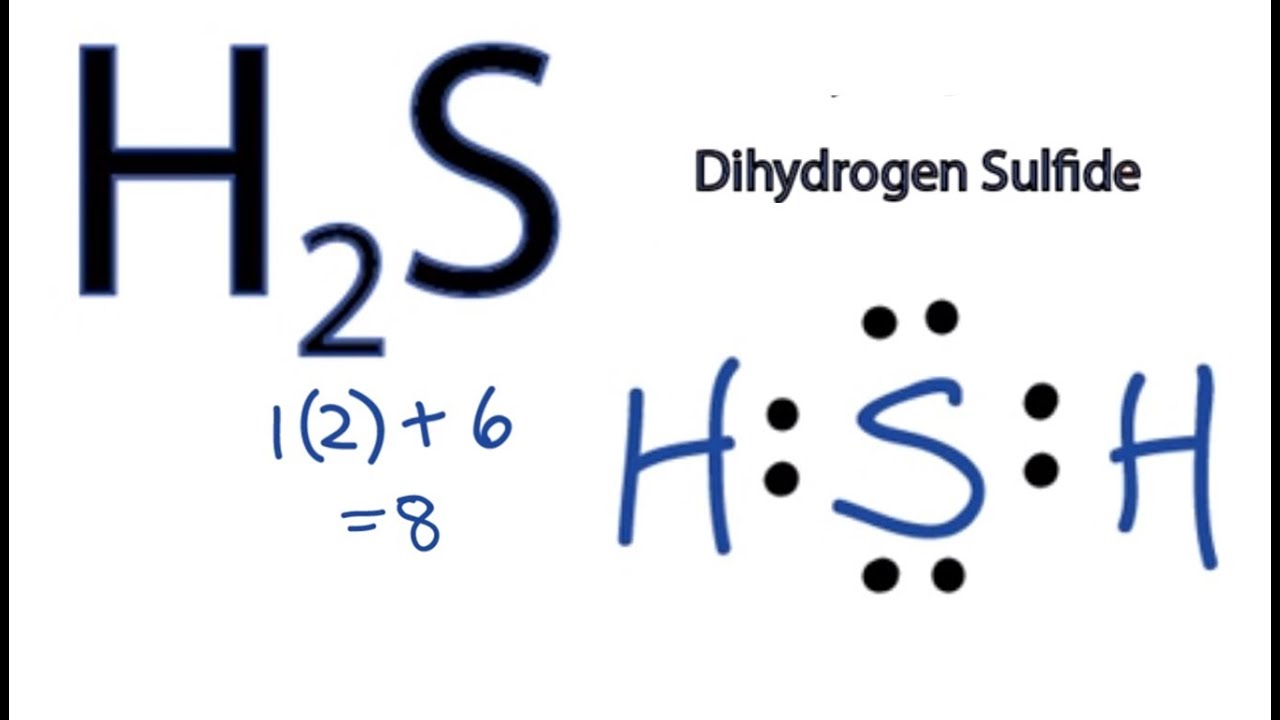
Estructura De Lewis H2s Estudiar
Introduction. Natural gas that contains more than 4 ppmv of hydrogen sulphide (H2S) is commonly referred to as "sour". This is because the odour of hydrogen sulphide gas in air at very low concentrations is similar to that of rotten eggs. Significant quantities of natural gas resources around the world are known to contain H2S.
H2S at emaze Presentation
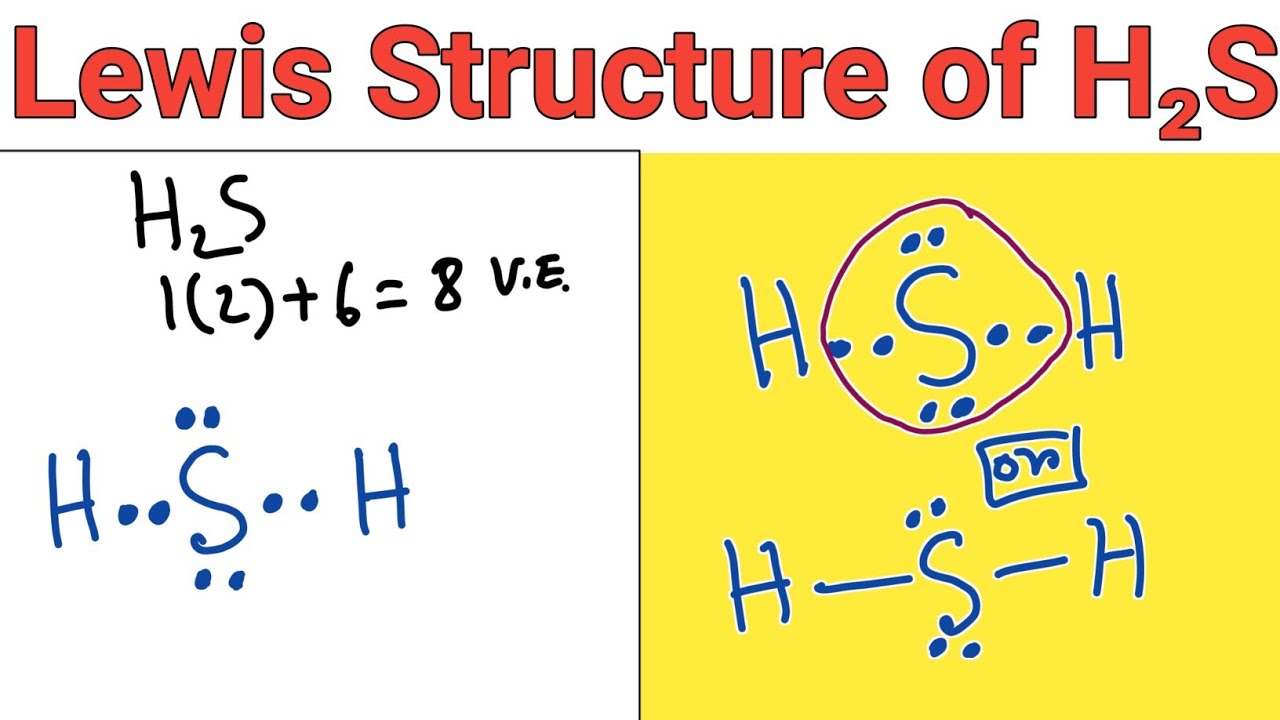
Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing. We'll put Sulfur here.

Estructura De Lewis H2s Estudiar
Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur needs t.

How to draw H2S Lewis Structure? Science Education and Tutorials
Struktur H2S Lewis memiliki atom sulfur (S) di tengahnya yang dikelilingi oleh dua atom hidrogen (H). Terdapat 2 ikatan tunggal antara atom belerang (S) dan masing-masing atom hidrogen (H). Terdapat 2 pasangan elektron bebas pada atom belerang (S). Jika Anda tidak memahami apa pun dari gambar struktur Lewis H2S di atas, tetaplah bersama saya.

H2S Molecular Geometry Science Education and Tutorials
What is the Lewis structure of hydrogen sulfide H 2 S?. Hydrogen sulfide H 2 S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. To draw the Lewis structure of hydrogen sulfide, follow these step-by-step instructions.

Lewis Structure Hydrogen Sulfide H2s Stock Vector (Royalty Free) 2264370065 Shutterstock
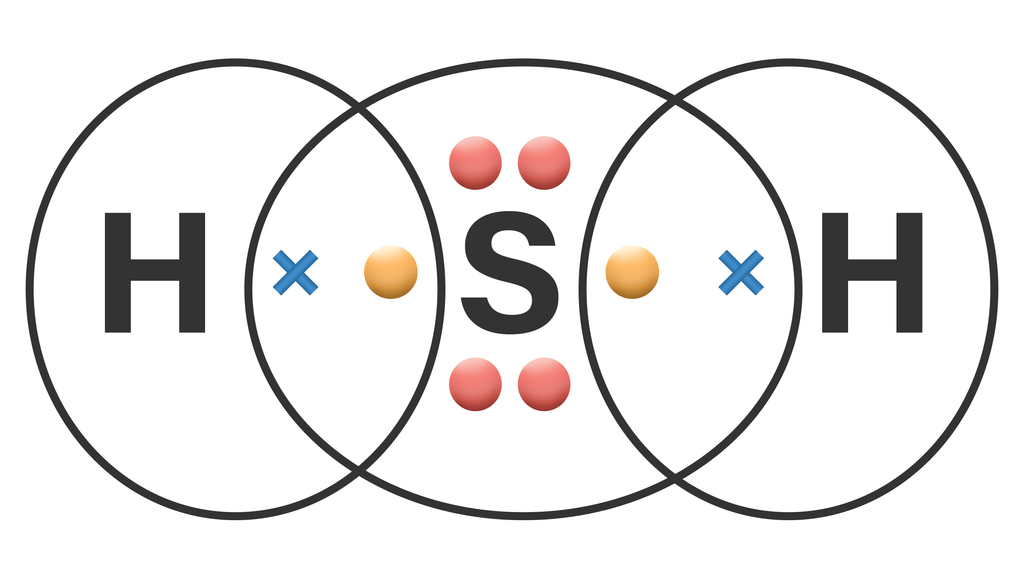
Now in the H2S molecule, you have to put the electron pairs between the sulfur atom (S) and hydrogen atoms (H). This indicates that the sulfur (S) and hydrogen (H) are chemically bonded with each other in a H2S molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom.
【4 Steps】H2S Lewis StructureLewis Structure for H2S (Dihydrogen Sulfide)Lewis Dot Structure
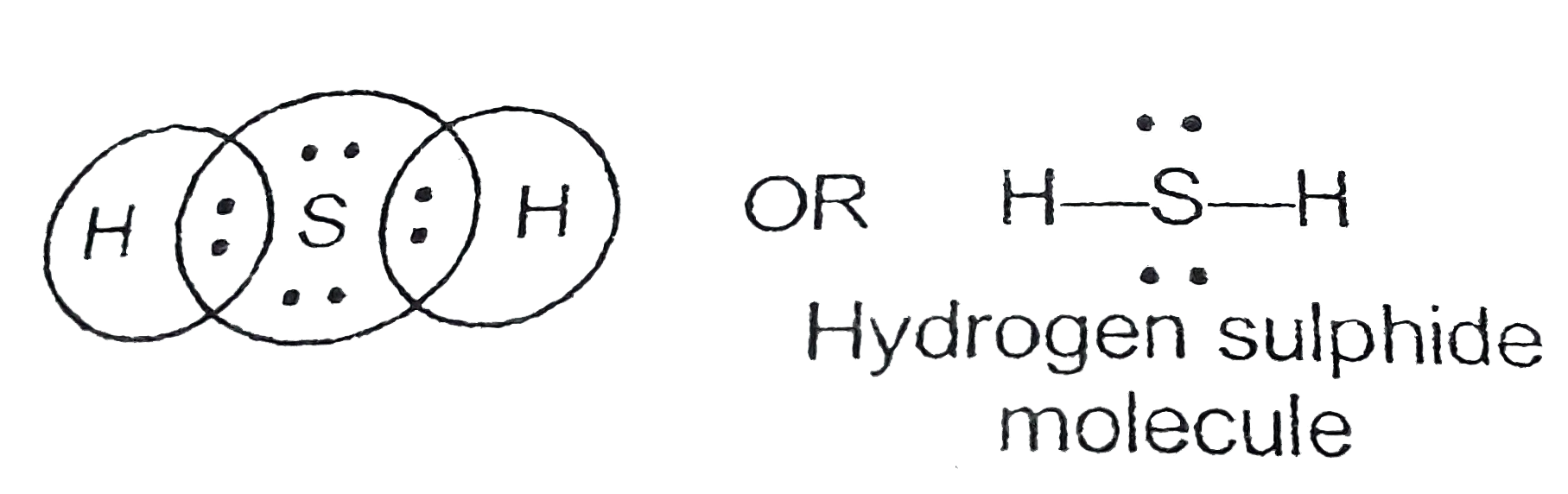
Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures ( LEDs ) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. [1] [2] [3] A Lewis structure can be drawn for any covalently.
Gambarkan struktur Lewis dari H2S...
A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide).The H2S Lewis structure is similar to the structure for water.
Lewis structure of H2S (Hydrogen sulphide) YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
The molar mass of H2S is 34.08 g/mol and its density is 1.363 g dm-3. The melting point and boiling point of H2S are -82℃ and -60℃ respectively. H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond.
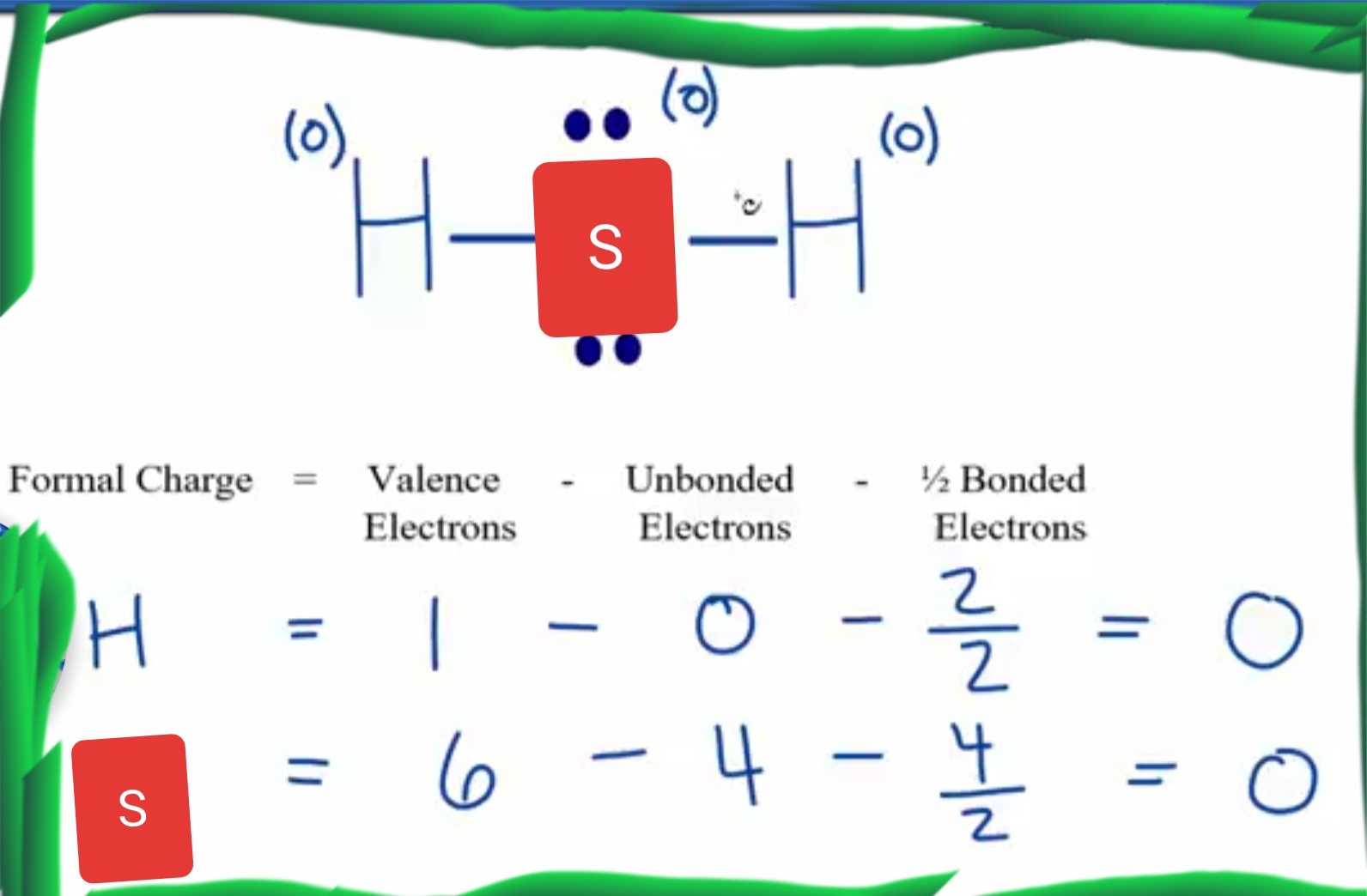
H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
H2S Lewis Structure: Understanding Covalent Bonding This article is a summary of a YouTube video "Lewis Structure of H2S, Hydrogen Sulfide" by chemistNATE TLDR The traditional understanding of sulfur's electron configuration and chemical bonding is challenged by the concept of non-metals bonding covalently.

Hydrogen sulfide h2s molecule skeletal formula Vector Image
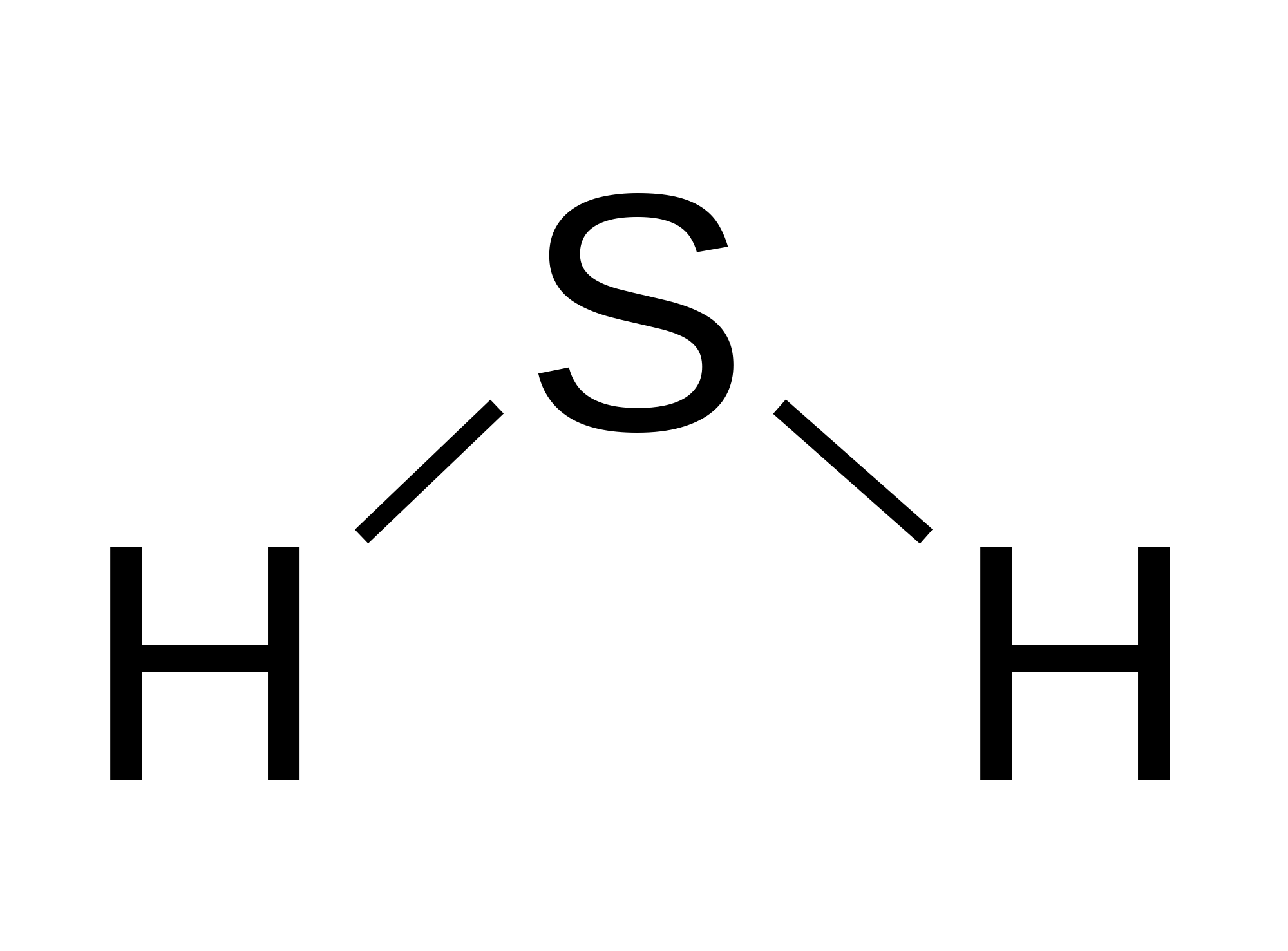
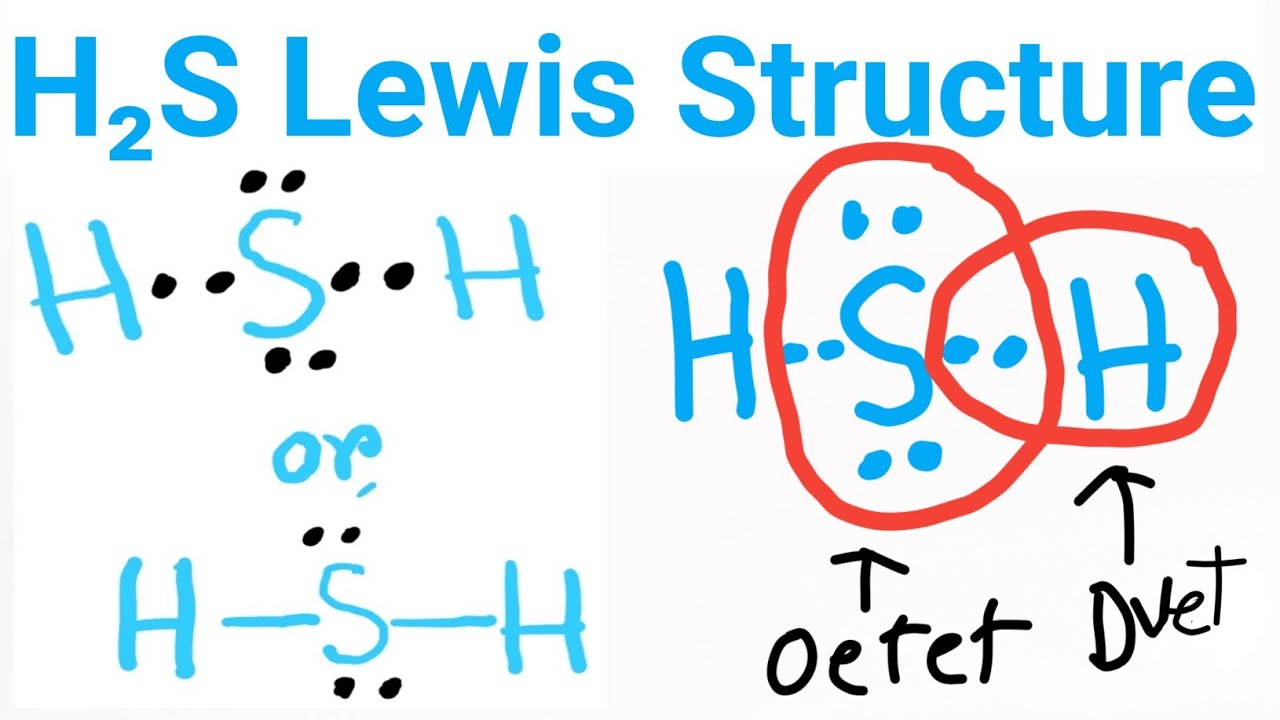
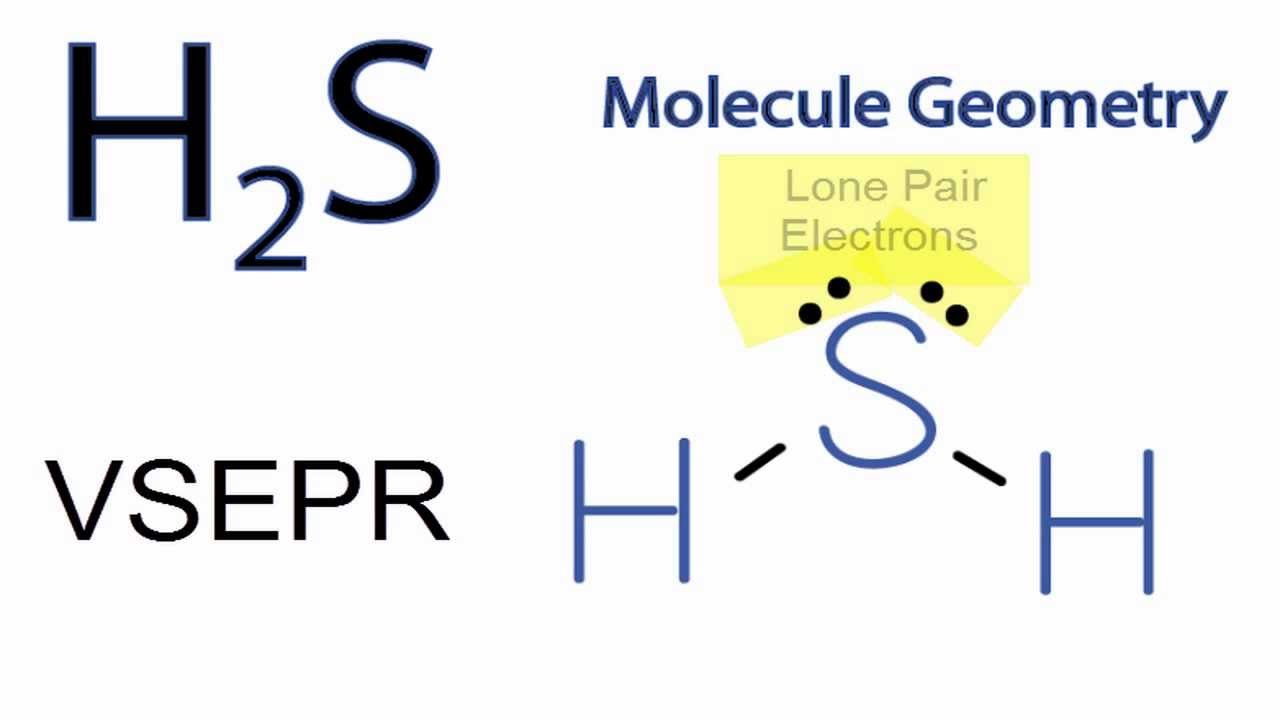
The Lewis structure of hydrogen sulfide is best represented as a bent H{eq}_2 {/eq}S molecule with two lone pairs of electrons on the S atom represented by two pairs of dots (or two bars).

H2s molecular geometry singleatila
The H2S Lewis structure represents the arrangement of atoms and electrons in the molecule. To draw the H2S Lewis structure, we start by identifying the valence electrons of hydrogen and sulfur atoms. Then, we place the atoms in a way that satisfies the octet rule, where each atom has eight electrons in its valence shell.
H2S Lewis Structure Lewis Dot Structure for H2S Hydrogen sulfide Lewis Structure YouTube
Hydrogen sulfide (H2S) consists of two hydrogen (H) atoms and one sulfur (S) atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Step-by-Step Guide to Drawing the Lewis Structure of H2S 1. Determining

H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial.
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
H2s lewis structure angle. In the H2S lewis structure, the intermixing 3s, 3p orbital form sp3 hybridized orbital, so the covalent bond angle should be 109.5̊ but it is lowered to 92.1̊ by the steric repulsion between dense two non bonding electron pair of 'S'. ad. For decreasing the bond angle (angle between the overlapping bonding.