
What is the Lewis Dot structure for CO2 (Carbon dioxide)?
1.2.1 Lewis Structure of Diatomic Molecules. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here: Figure 1.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine
:max_bytes(150000):strip_icc()/CO2LewisStructure-591c94063df78cf5fadfde77.png)
Lewis Structure Definition and Example
Steps of drawing CO2 lewis structure Step 1: Find the total valence electrons in CO2 molecule. In order to find the total valence electrons in CO2 (carbon dioxide) molecule, first of all you should know the valence electrons present in carbon atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
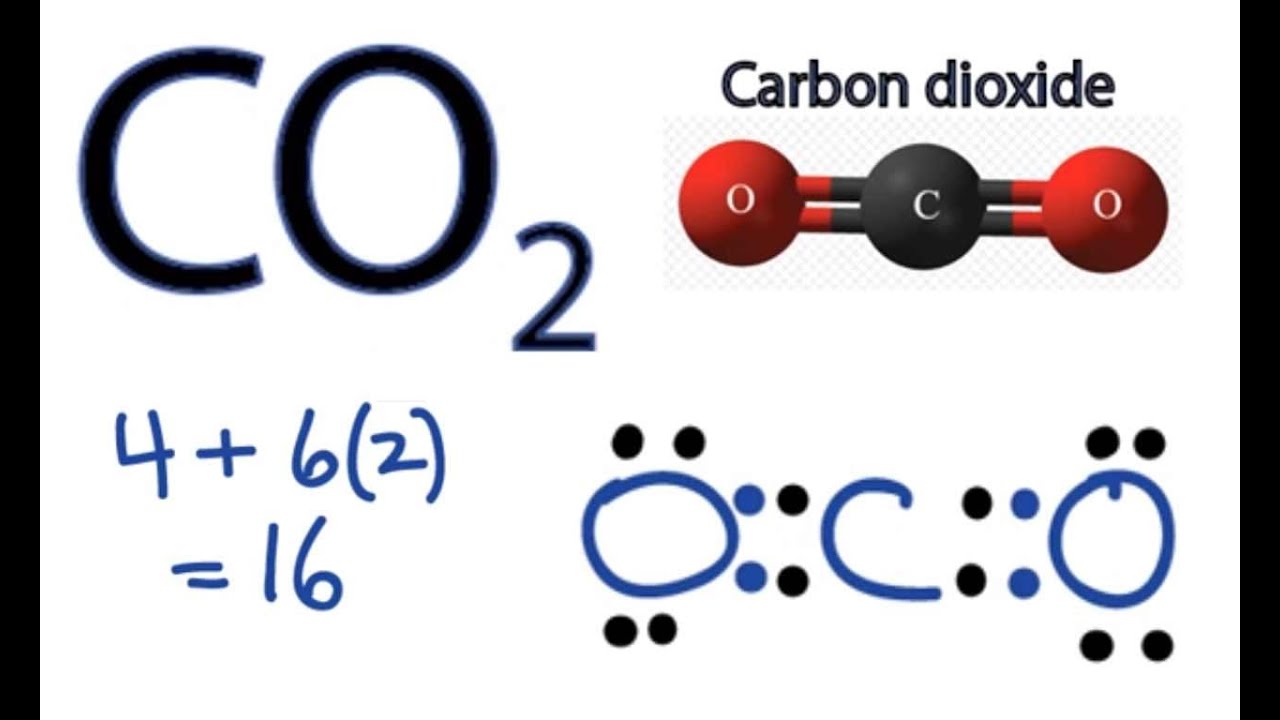
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide YouTube
For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. To further understand the molecular geometry of CO2, let us quickly go through its hybridization.

CO2 Molecular Geometry Science Education and Tutorials
A green carbon capture and conversion technology offering scalability and economic viability for mitigating CO 2 emissions is reported. The technology uses suspensions of gallium liquid metal to reduce CO 2 into carbonaceous solid products and O 2 at near room temperature. The nonpolar nature of the liquid gallium interface allows the solid products to instantaneously exfoliate, hence keeping.

Vector Illustration Lewis Structure Carbon Dioxide Stock Vector (Royalty Free) 2232946047
Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO2 (carbon dioxide). In order to draw the lewis structure of CO2, first of all you have to find the total number of valence electrons present in the CO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

CO2 Lewis Structure, Molecular Geometry, Molar Mass & Hybridization
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.

CO2 (Carbon Dioxide) Lewis Dot Structure Science Trends
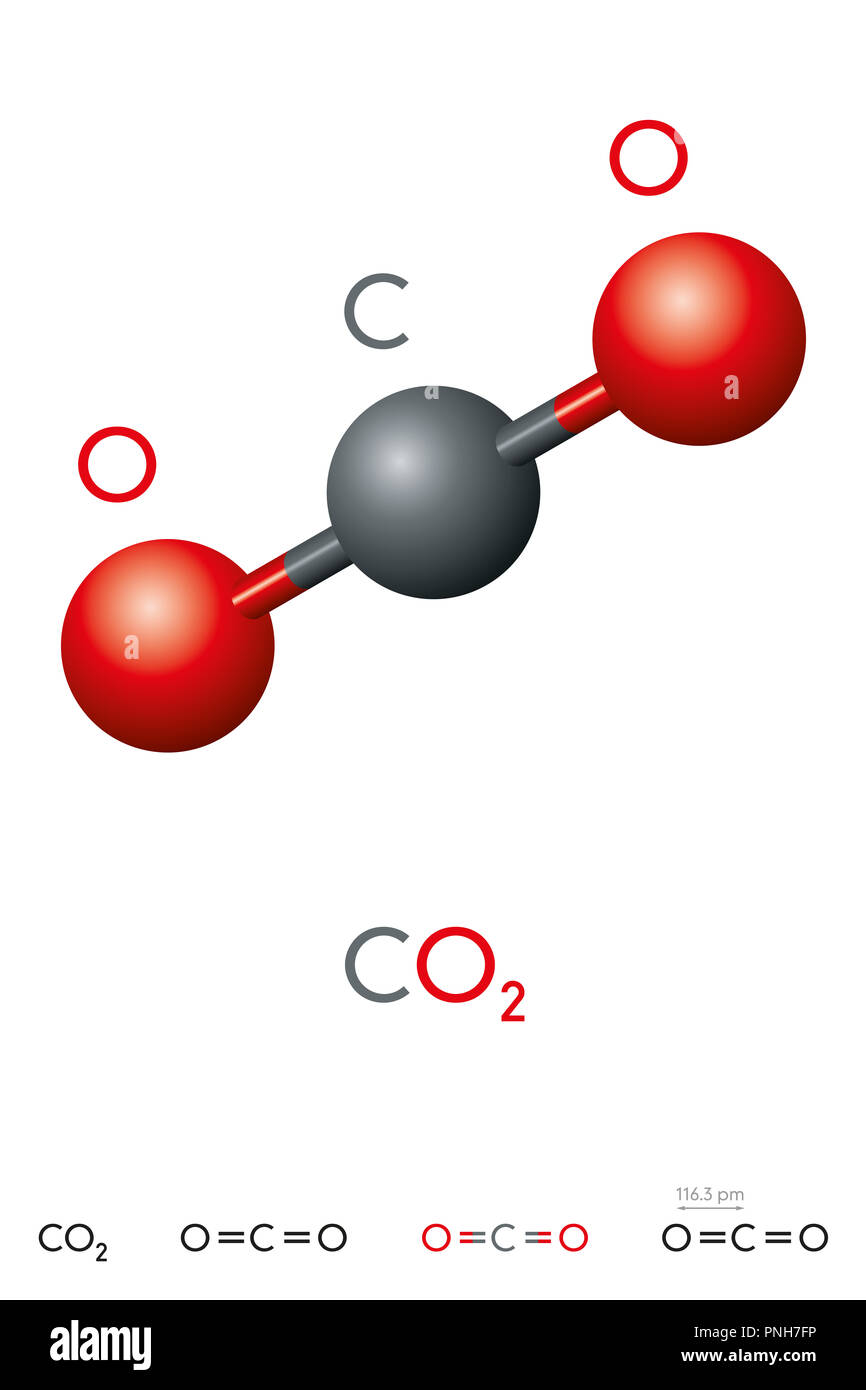
Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial.
Carbon dioxide, CO2, molecule model and chemical formula. Carbonic acid gas. Colorless gas. Ball
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

CO2 Lewis Structure, Drawing Method of CO2 Lewis Structure, Molecular Geometry of CO2
Therefore it is put in the center of the dot structure. For the CO 2 Lewis structure there are a total of 16 valence electrons available. Transcript: OK, this is Dr. B. We're going to do the Lewis structure for CO2, Carbon dioxide. On the periodic table, Carbon is in group 4, or 14 sometimes; and then Oxygen is in group 6 or 16.

CO2, Struktur Lewis, Bentuk Molekul, dan Kepolarannya YouTube
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.

Carbon Dioxide Lewis Structure How to Draw the Lewis Structure for Carbon Dioxide YouTube
CO2 has a linear molecular geometry with a bond angle of 180° on a plan. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. Carbon dioxide has an sp hybridization type because the steric number of central carbon is 2. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds.

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Techiescientist
To draw the Lewis structure of CO2, we first need to determine the number of valence electrons in each atom. Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. In total, CO2 has 16 valence electrons. Next, we need to arrange the atoms in the molecule. Carbon is the central atom in CO2, with each oxygen atom bonded.

MENGGAMBAR STRUKTUR LEWIS CO2BANK SOAL KE 9 YouTube
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or Nonpolar,Octet Rule
Carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. The carbon-oxygen ratio in a CO 2 molecule is 1:2. Two double bonds connect the carbon and oxygen atoms in the Lewis structure. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom.

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or Nonpolar,Octet Rule
These electrons push away the bonded pairs of electrons,giving it its "V shape". In the case of carbon dioxide,however,the carbon atom has no lone pairs so there is no repulsion between the bonded pairs and lone pairs. For the second part of your question, the electrons are present in a 3D region around the nucleus of an atom.

Lewis Structure Carbon Dioxide Co2 เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 2109748577 Shutterstock
I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles.