7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts
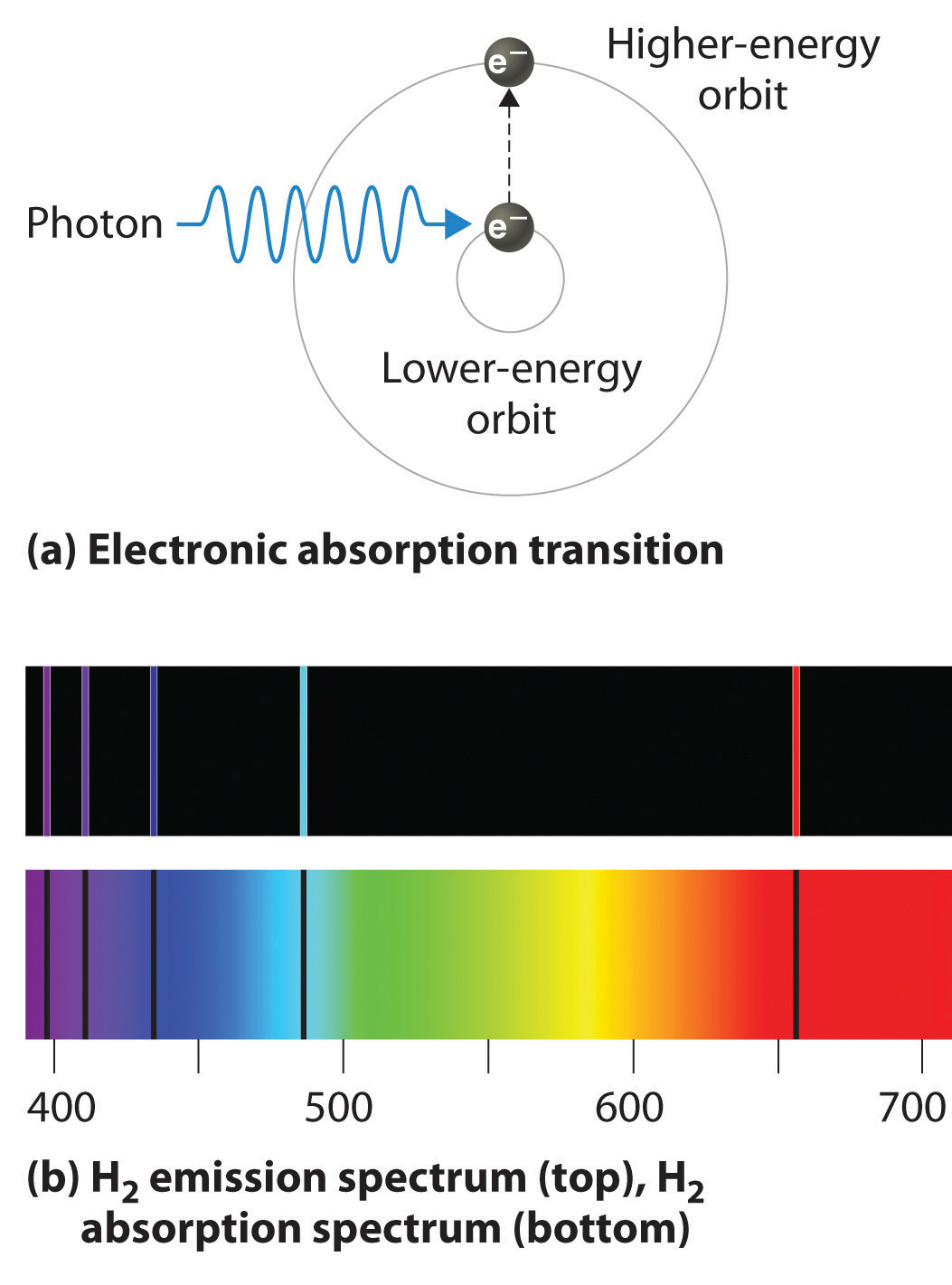
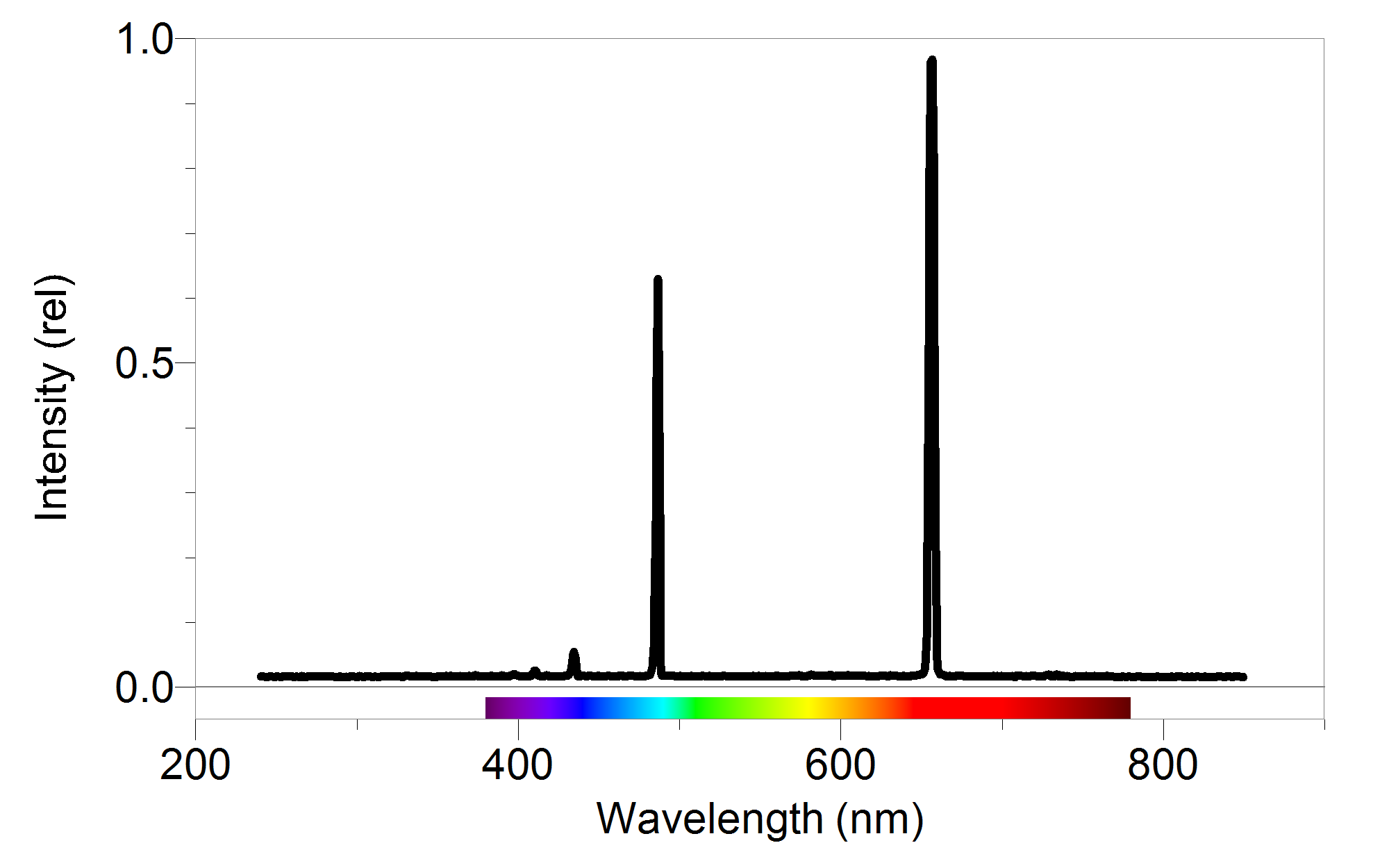
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm. Figure 7.3.1: The Emission of Light by Hydrogen Atoms. (a) A sample of excited hydrogen atoms emits a characteristic red light.

Hydrogen Spectrum// Atomic structure . YouTube
Recall the equation above: The energy gap between the ground state and the point at which the electron leaves the atom can be determined by substituting the frequency and looking up the value of Planck's constant from a data book. (4) Δ E = h ν (5) = ( 6.626 × 10 − 34) ( 3.28 × 10 15) (6) = 2.173 × 10 − 18 J.

The Bohr Atom Chemwiki
SPEKTRUM ATOM HIDROGEN Toni Dwi Fauzi (ACB 115 003) email : [email protected] PENDAHULUAN Hidrogen merupakan atom yang memiliki struktur paling sederhana, dibandingkan dengan atom lain. Hidrogen terdiri atas 1 elektron dan 1 proton. Hidrogen banyak dijadikan dasar bagi ilmuwan dalam mempelajari atom, karena strukturnya yang sangat sederhana.
Hydrogen Atomic Spectrum مصادر الكيمياء
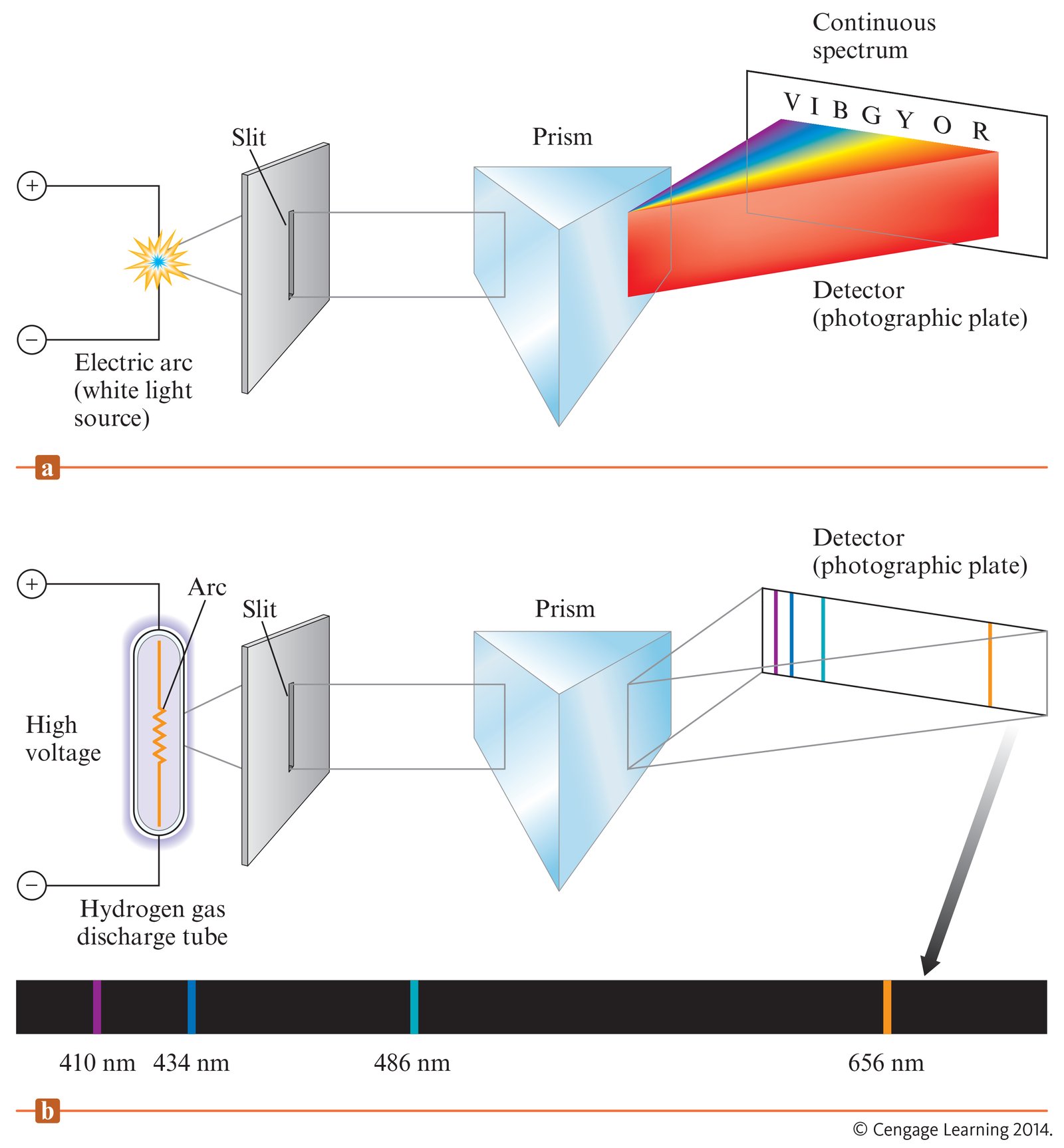
Balmer decided that the most likely atom to show simple spectral patterns was the lightest atom, hydrogen. Ångström had measured the four visible spectral lines to have wavelengths 656.21, 486.07, 434.01 and 410.12 nm (Figure 1.4.4 ). Balmer concentrated on just these four numbers, and found they were represented by the phenomenological formula:

Hydrogen Hydrogen Line Spectra
Simak penjelasan singkat mengenai spektrum atom hidrogen yang dilengkapi dengan contoh soal dan pembahasannya.Kunjungi juga website kami di https://tanya-tan.
Hydrogen spectrum Chemistry, Class 11, Structure Of Atom
The gas company, along with the Los Angeles Department of Water and Power, Mitsubishi Power, and others, is part of an organization that seeks to deliver green hydrogen to the Los Angeles basin at.
Line Spectrum Of Hydrogen Modelo Atómico de Bohr Características y Postulados Lifeder / Our
Spektrum atom hidrogen pada materi ini termasuk kedalam spektrum garis emisi. DASAR TEORI A. Percobaan Spektrum Atom Hidrogen Percobaan untuk mengamati spektrum atom hidrogen dapat dilakukan dengan cara memberikan tegangan listrik pada tabung sinar yang berisi gas hidrogen dengan tekanan rendah. Apabila atom diberi energi (dalam hal ini.
Spectrum of Atomic Hydrogen > Experiment 21 from Advanced Physics with Vernier — Beyond Mechanics
The Spectrum of Atomic Hydrogen. For almost a century light emitted by the simplest of atoms has been the chief experimental basis for theories of the structure of matter. Exploration of the hydrogen spectrum continues, now aided by lasers. by Theodor W. Hansch, Arthur L. Schawlow and George W. Series. The spectrum of the hydrogen atom has.

The Emission Spectrum of the Hydrogen Atom YouTube
SPEKTRUM ATOM HIDROGEN. Toni Fauzi. PENDAHULUAN Hidrogen merupakan atom yang memiliki struktur paling sederhana, dibandingkan dengan atom lain. Hidrogen terdiri atas 1 elektron dan 1 proton. Hidrogen banyak dijadikan dasar bagi ilmuwan dalam mempelajari atom, karena strukturnya yang sangat sederhana, termasuk dalam mempelajari spektrum atom.

PPT ATOM dan MOLEKUL PowerPoint Presentation, free download ID1150071
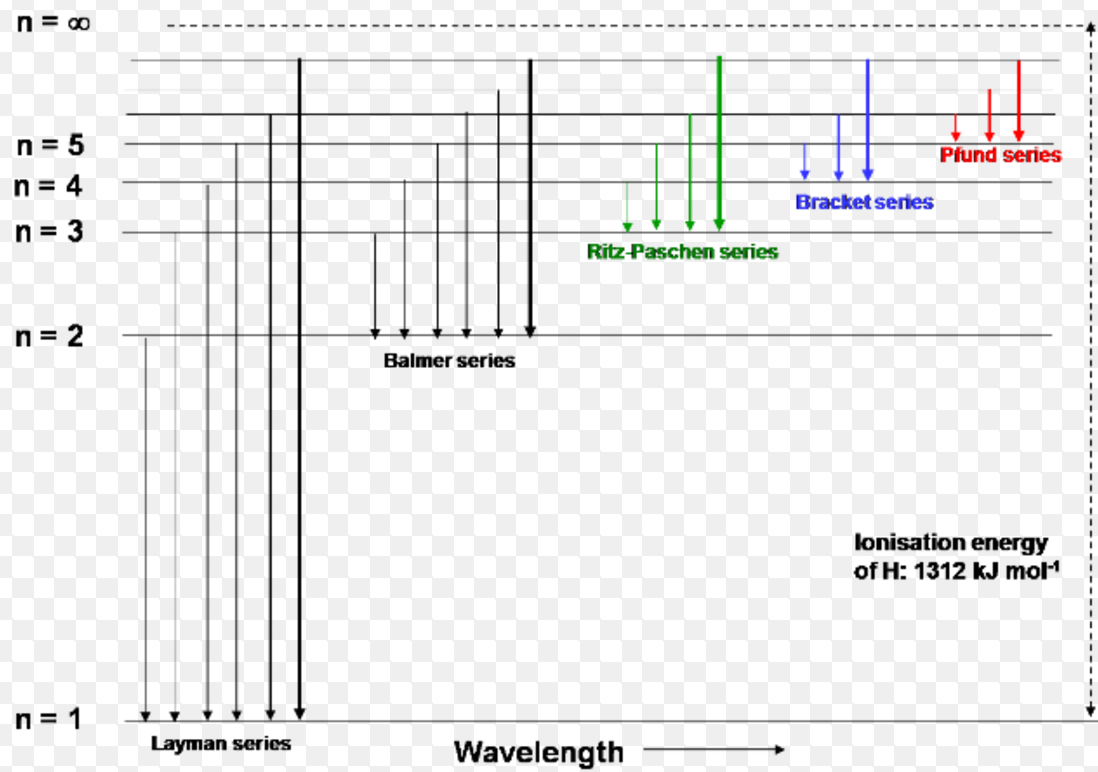
The spectral series of hydrogen, on a logarithmic scale.. The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula.These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development.
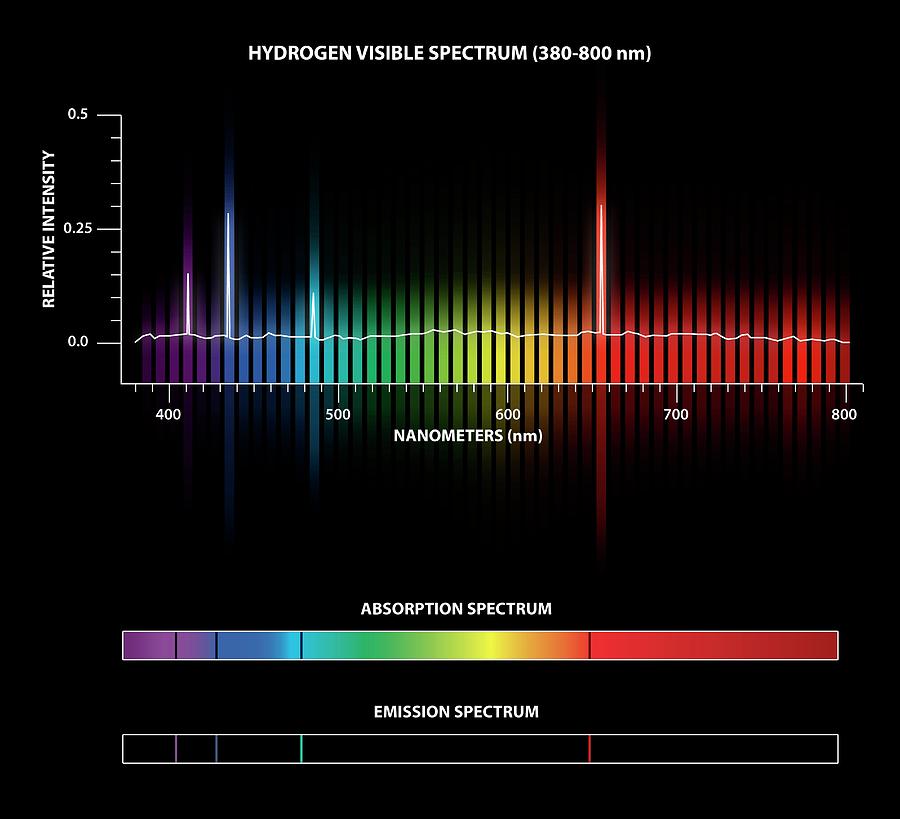
Absorption Spectra Of Hydrogen
You have no doubt been exposed many times to the Bohr model of the atom. You may have even learned of the connection between this model and bright line spectra emitted by excited gases. In this experiment, you will take a closer look at the relationship between the observed wavelengths in the hydrogen spectrum and the energies involved when electrons undergo transitions between energy levels.
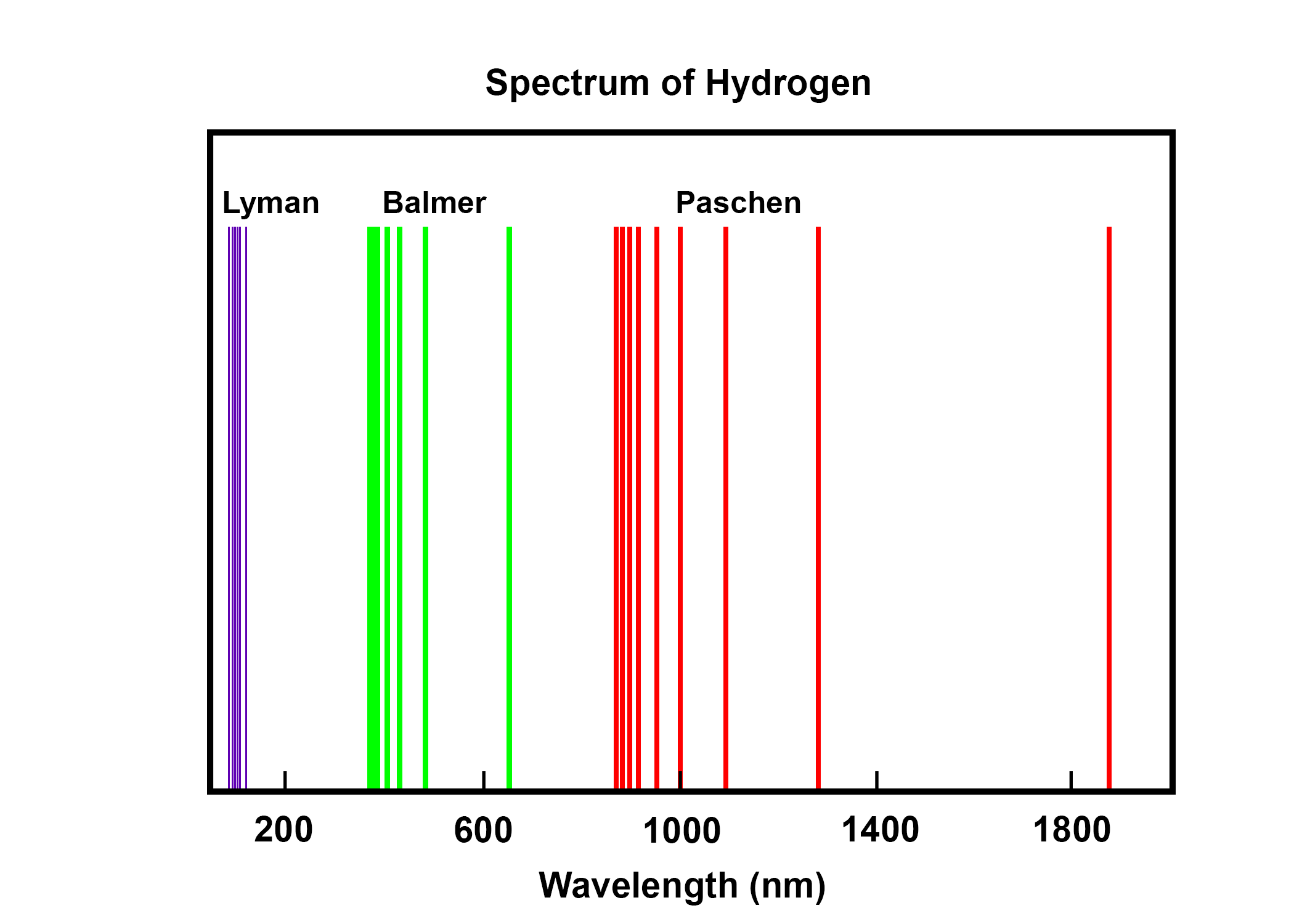
Hydrogen atom, spectrum and electron transitions
Ketika Bohr membangun teori atom hidrogen, ada satu hasil eksperimen yang penting terkait dengan atom hidrogen. Eksperimen tersebut adalah pengamatan spektrum garis yang dipancarkan oleh atom hidrogen. Salah satu spektrum yang terkenal diperlihatkan pada Gambar 280.1. Spetrum tersebut membentuk deret garis dengan warna berubah dari ungu ke merah. Deret spektrum garis tersebut dinamakan deret.

Evolusi Model Atom Spektrum Atom Hidrogen (Fisika SBMPTN, UN, SMA) YouTube
Model Atom Hidrogen - Mekanika Kuantum, Atom Hidrogen, Model Bohr - PhET. Lompat ke Isi Utama.
/GettyImages-1096547948-35b3799817ca4b2fa06888893ef4a348.jpg)
Visible Line Spectrum Of Hydrogen
Latihan Soal Atom Hidrogen (Sukar) Nilai panjang gelombang 4 spektrum pertama pada deret Balmer yang tepat (dalam nm) adalah.. Belajar Modul, Rumus, & Soal Atom Hidrogen dan kuis interaktif. Dapatkan Modul, Rumus, & Soal Atom Hidrogen lengkap di Wardaya College.

2.2 The Line Spectrum of Hydrogen [SL IB Chemistry] YouTube
Spektrum Atom Hidrogen. Atom Hidrogen adalah atom yang paling sederhana. Dalam sebuah tabung lucutan gas diberi beda potensial yang tinggi, sehingga terjadi lucutan muatan listrik. Gas hidrogen menjadi bercahaya dan memancarkan cahaya merah kebiru-biruan. Cahaya ini dapat dianalisis dengan sebuah spektrograf (alat untuk menyelidiki spektrum).
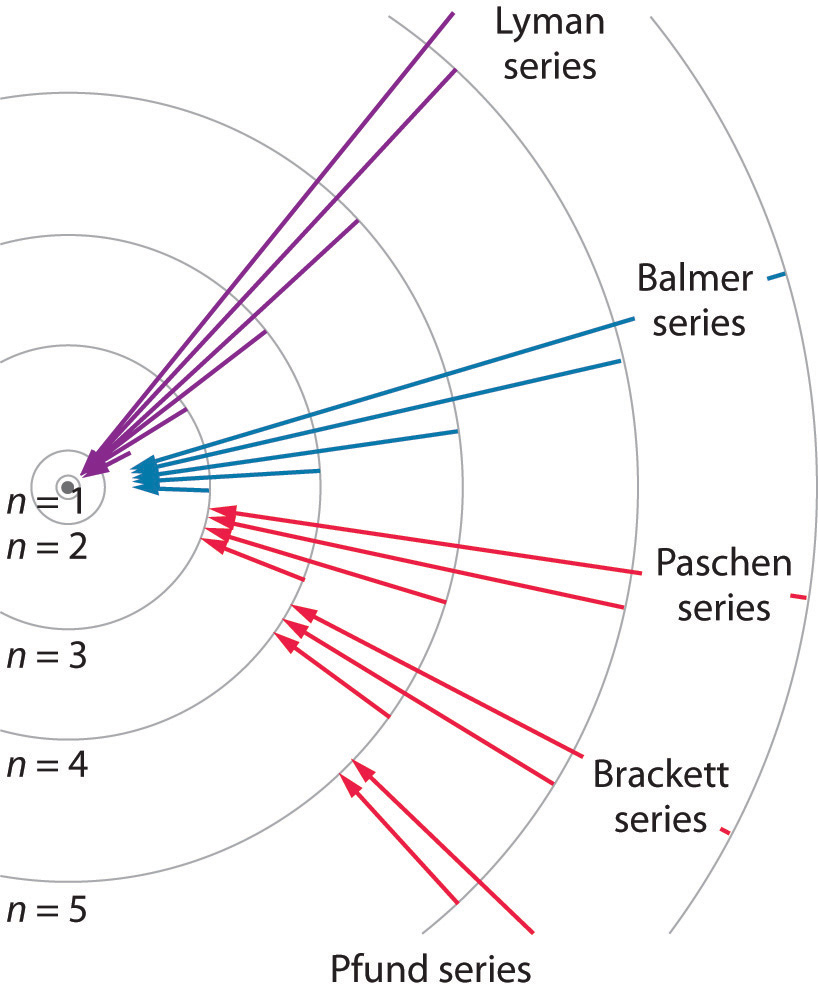
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts
Scientists at CERN found a way to trap hydrogen's mirror twin, antihydrogen, long enough to study it in greater detail than ever before.