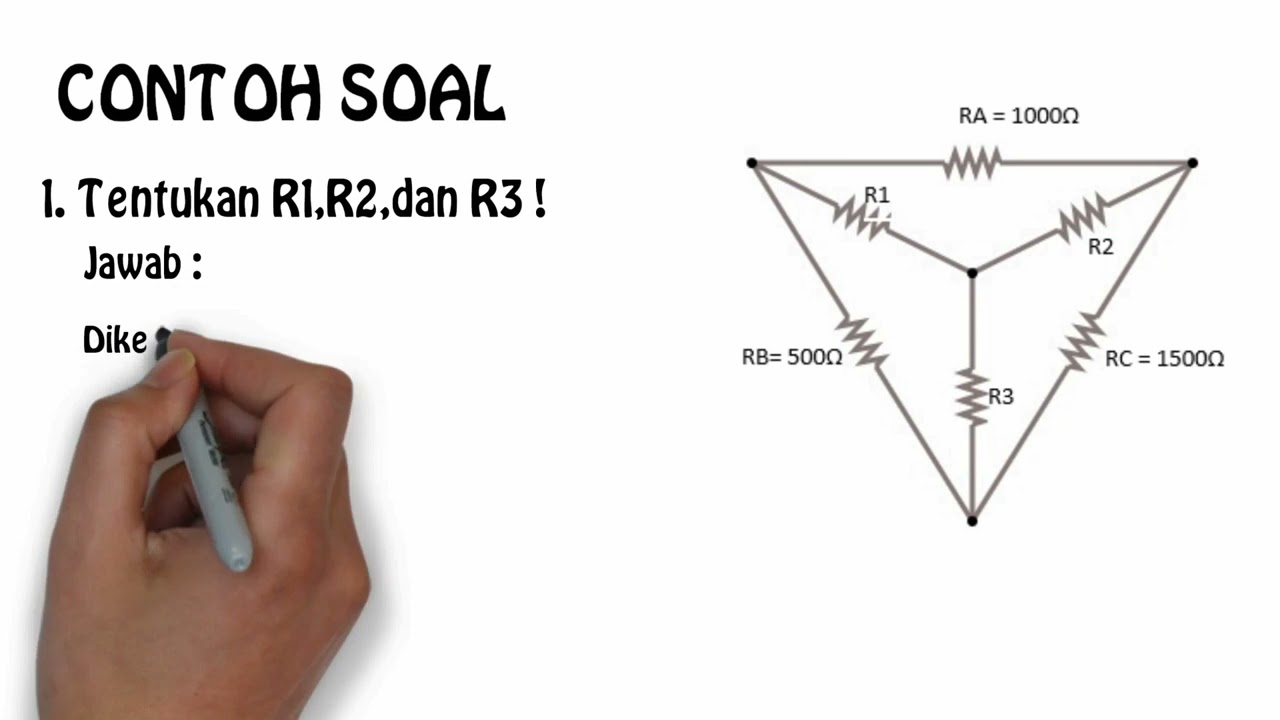
cara menghitung rumus star delta kelistrikan YouTube
Modified by Joshua Halpern ( Howard University) 10.8: Relationships Involving ΔHrxn is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Hess's law argues that for a chemical reaction, the enthalpy of reaction (ΔHrxn) is the difference in enthalpy between products and reactants; the units of ΔHrxn.

Menentukan Delta H (TERMOKIMIA) KIMIA SMA YouTube
Verifying that you are not a robot.

Menentukan Entalpi Reaksi Berdasarkan Entalpi Pembentukan Serba Ada
The equation for determining the enthalpy of fusion ( ΔH Δ H) is listed below. ΔH = nΔHfus (1) (1) Δ H = n Δ H f u s. with. n n = number of moles. ΔHfus Δ H f u s the molar heat of the substance. Example 1 1. Calculate the heat when 36.0 grams of water at 113 °C is cooled to 0 °C.

3 Ways to Calculate the Enthalpy of a Chemical Reaction Wiki How To
Lancia Delta HF Integrale Evo II. 1993 to 1995. 2 for sale. CMB $105,345. There are 2 1994 Lancia Delta HF Integrale Evo II for sale right now - Follow the Market and get notified with new listings and sale prices.

Termokimia Pengertian Persaman Reaksi Rumus Dan Contoh Soal Soal
ΔH = -57.20 kJ. This value of ΔH is a combination of two physical effects. First, the internal energy of the chemicals decreases by 54.72 kJ when two moles of NO 2 combine to form one mole of N 2 O 4. Second, the value of PV decreases by 2.478 kJ when two moles of NO 2 combine to form one mole of N 2 O 4.

√ Rumus Delta T
1. Entalpi Pembentukan Standar ( ΔH ∘ f) Perubahan entalpi standar pada pembentukan 1 mol zat langsung dari unsur unsurnya pada keadaan standar (298 K, 1 atm). Sebagai contoh entalpi pembentukan standar untuk air adalah − 285 kJ/mol, maka persamaan termokimianya adalah sbb: H2(g) + 1 2O2 → H2O(l) ΔH = − 285 kJ.
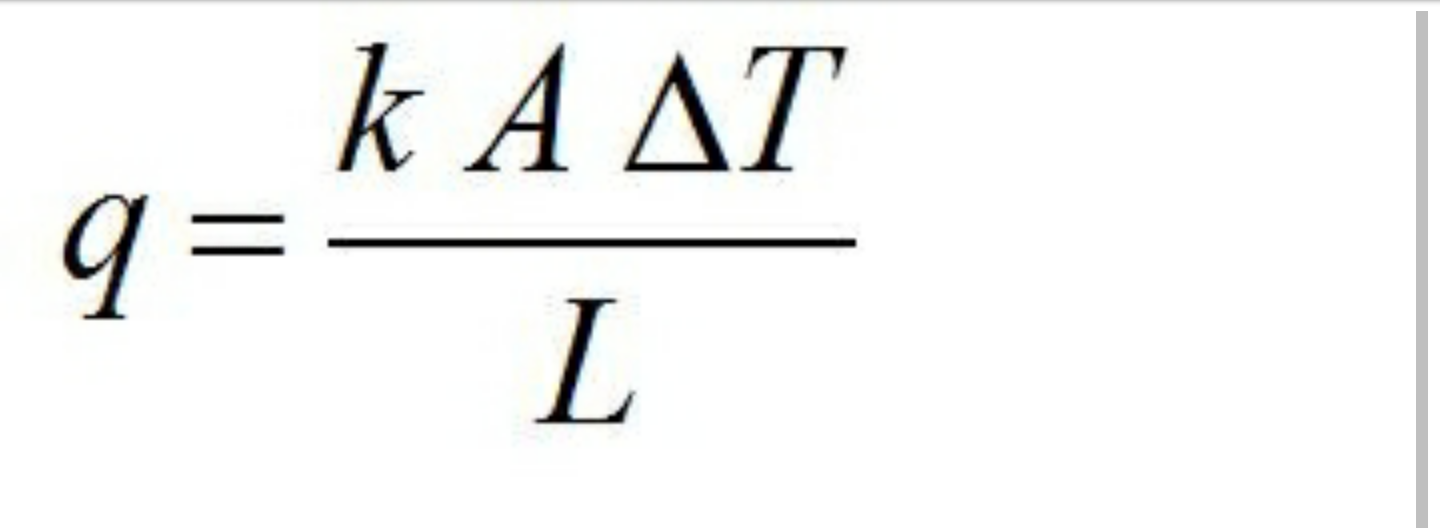
√ Rumus Delta T
Gunakan rumus ∆H = m x s x ∆T untuk menyelesaikannya. Jika kamu sudah memiliki m, massa reaktan, s, kalor jenis produk, dan ∆T, perubahan suhu reaksi, maka kamu sudah siap untuk mencari entalpi reaksi. Masukkan nilaimu ke dalam rumus ∆H = m x s x ∆T dan kalikan untuk menyelesaikannya. Jawabanmu ditulis dalam satuan energi, yaitu Joule.

Contoh Soal Hukum Hess dengan menggunakan data delta Hf (di akhir ada
Berikut penjelasan kelompok sifat koligatif: 1. Koligatif Larutan - Penurunan tekanan uap jenuh (Δ Tp ) Penurunan tekanan uap jenuh adalah selisih tekanan uap pelarut murni dan tekanan uap larutan . tabel penurunan tekanan uap jenuh larutan non elektrolit dan elektrolit ↓. Uraian. Larutah non elektrolit.

Rumus Perubahan Energi Dalam Tulisan
Step 1: Set Up the Equation. Arrange your given ΔHf and ΔH values according to the following equation: ΔH = ΔHf (products) - ΔHf (reactants). For example, imagine that you want to know ΔHf for acetylene, C 2 H 2, for the reaction C 2 H 2 (g) + (5/2)O 2 (g) --> 2CO 2 (g) + H 2 O (g), the combustion of acetylene, the ΔH of which is -1,256.

Persamaan Termokimia Kimia SMA YouTube
Dapatkan Modul, Rumus, & Soal Perhitungan Entalpi Reaksi lengkap di Wardaya College.. $\Delta H=\sum\Delta H_{f}\mbox{ produk}-\sum\Delta H_{f}\mbox{ pereaksi}$ Energi Ikat Rata-Rata. Reaksi kimia antar molekul dapat dianggap berlangsung dalam dua tahap, yaitu pemutusan ikatan pada pereaksi dan pembentukan ikatan pada produk.

Delta H Reaksi Data ΔHf Materi Termokimia Kimia SMA Pojan.id
Standard Enthalpies of Reaction. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\Delta{H_f^o}\) values are known. The standard enthalpy of reaction \(\Delta{H_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states.
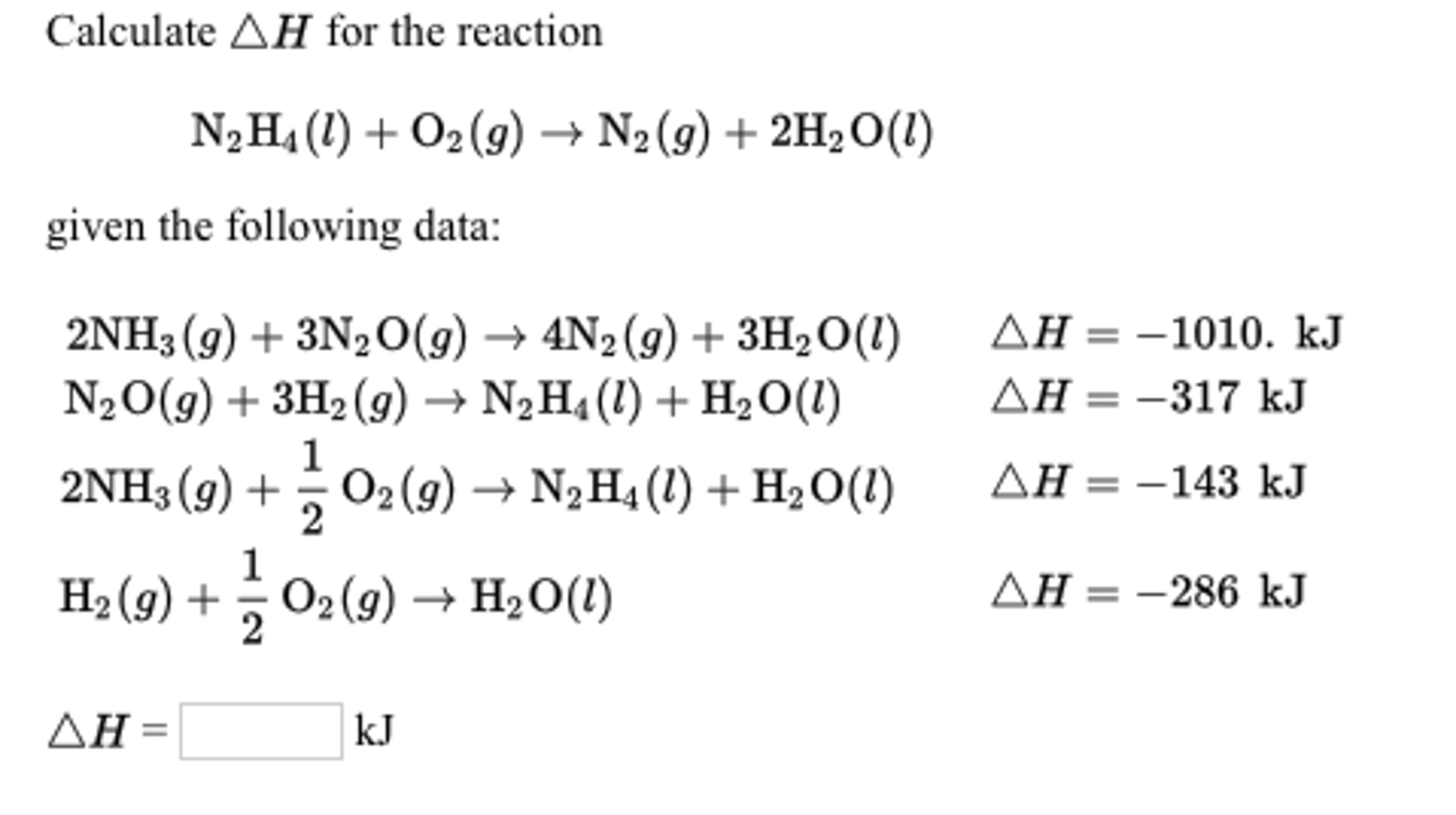
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere
cara mudah menentukan delta H reaksi ata entalpi reaksi pada reaksi pembentukan 2C+3H2 menjadi C2H6 jika diketahui delta H reaksi masing-masing unsur atau se.

Rumus Delta S Kimia Bit CDN
HOW DO YOU CALCULATE Δ H (DELTA H)? Once you memorized the relationship between the side the energy is on in a chemical equation, the Δ H and endothermic or exothermic, you are probably curious about how Δ H is calculated. It is calculated by the total energy contained in the molecules of products minus the total energy of the molecules in.

Diketahui delta Hf CH4O(l)=238,6 kJ/mol delta Hf CO2(g)...
2. I have seen the energy of a photon given by the formulas: E = h ⋅ f (1) (1) E = h ⋅ f. Where E E = energy of the photon, h h = Planck's constant, f f = frequency of radiation (Source: BBC article) I've also seen it given as. E = h ⋅ ν (2) (2) E = h ⋅ ν. Where ν ν stands for frequency (Source: Wikipedia article) But in this topic.
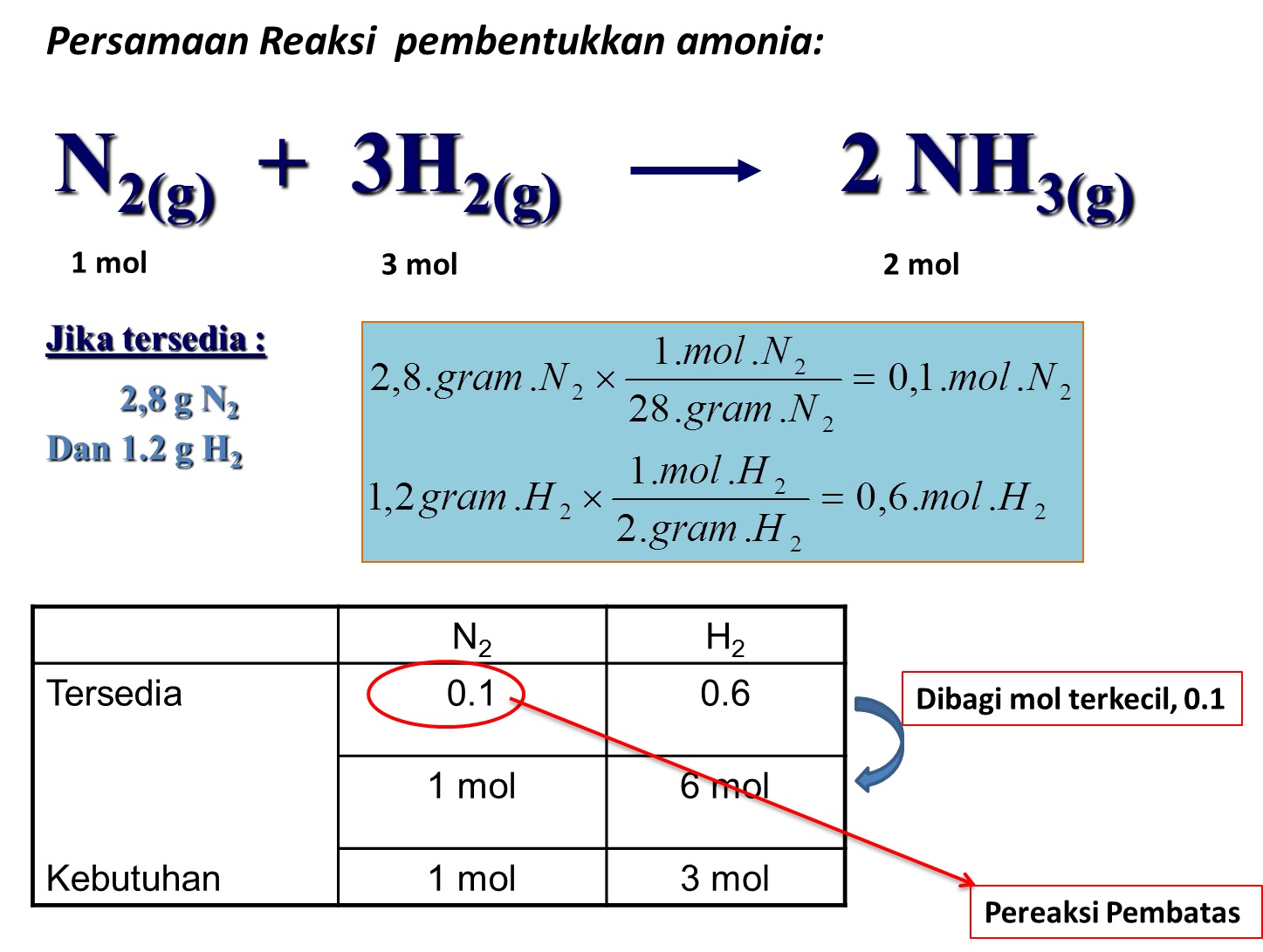
5 Tahapan Reaksi Kimia kabarmedia.github.io
Lancia Delta HF Integrale Evo II (1993 to 1995) CMB $104,226. . FOLLOW MARKET. Presented in June 1993, the final major version of the Delta HF Integrale became known as the Evo II. Compared to its predecessor, the Evo 1, it featured an updated version of the 2-litre 16-valve turbo engine, producing 215 PS (158 kW; 212 hp) and maximum torque of.
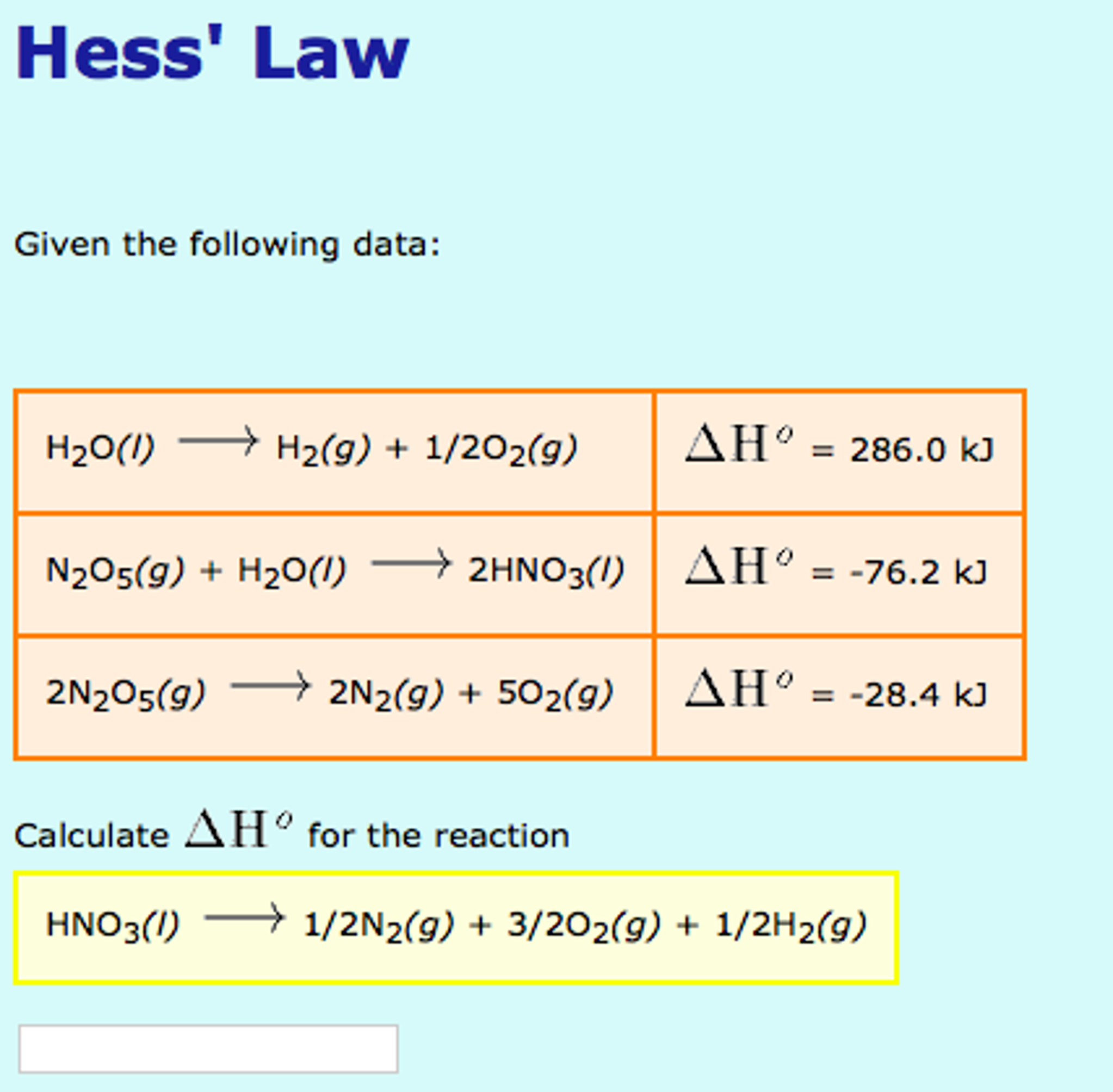
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere
Rumus yang digunakan Rumus/persamaan yang akan kita gunakan adalah: ΔH = ΣΔH f produk - ΣΔH f reaktan: Penjelasan:. Menghitung perubahan entalpi (delta Hf) Catatan: Mohon dimaklumi, jika materi dalam tutorial ini dirasa kurang lengkap dan menyeluruh.