
DynaGlo Pro 60K BTU Forced Air Propane Portable HeaterRMCFA60DGP The Home Depot
Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom.

Products Thermo Scientific™ LLysine2HCl For SILAC, 50 Mg
Since there is an equal number of each element in the reactants and products of (2Na) + 2HCl = 2NaCl + H2, the equation is balanced. Balance (2Na) + HCl = NaCl + H2 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction.
Products Thermo Scientific™ LLysine2HCl, 13C6, 15N2 For SILAC, 50 Mg
Reaksi propuna dengan HCl merupakan reaksi adisi alkuna yang akan menghasilkan senyawa alkena bersubstituen halida. Senyawa propuna merupakan alkuna asimetris atau senyawa alkuna yang ikatan rangkapnya terletak di antara atom C yang mengikat substituen berbeda. Pada adisi alkena asimetris oleh asam halida, akan berlaku aturan Markovnikov yaitu.

C3H4 Hcl Sinau
Molecular formula: C9H15N5O3 × 2HCl Formula weight: 314.17. [a]D = -6.6° to -7.6° (C = 1 in 0.1 N HCl). EM (216 nm) = 15,400 (0.1 N HCl)1 EM (264 nm) = 13,750 (0.1 N HCl)1. Tetrahydrobiopterin is a natural cofactor for phenylalanine hydroxylase,2 tyrosine hydroxylase,3 tryptophan hydroxylase,4 nitric oxide synthase,5and alkylglycerol.

11KG Propane Gas Cylinder Propane for Mobile Catering and Caravans Flogas
Since there is an equal number of each element in the reactants and products of Pb + 2HCl = PbCl2 + H2, the equation is balanced. Balance Pb + HCl = PbCl2 + H2 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as.
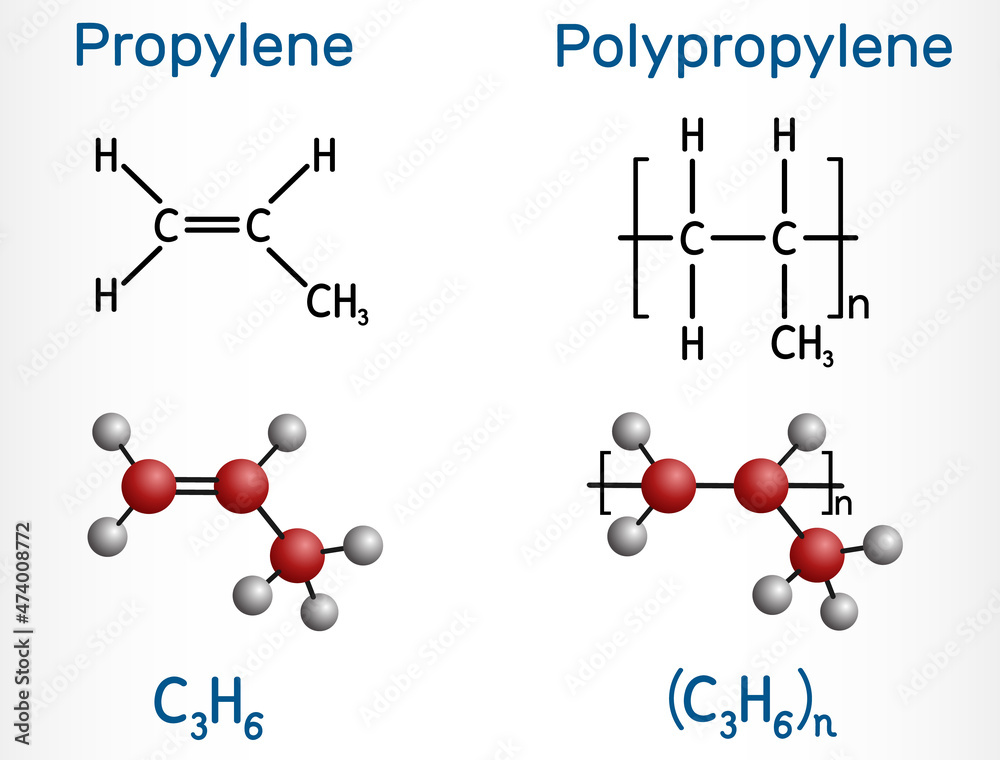
Propylene (propene) and polypropylene (PP, polypropene) molecule. Polymer and monomer
The reaction, represented by the equation H 2 + Cl 2 → 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture. Hydrogen chloride is commonly prepared both on a laboratory and on an industrial scale by the reaction of a chloride, generally that of sodium (NaCl), with sulfuric acid (H 2 SO 4).
APExBIO H 89 2HClPotent PKA inhibitorCAS 130964395
2,331 of the 14,158 available properties in Los Angeles are house rental.They account for nearly half of the properties in this destination (47.19%).Additionally, the average size of house rental in Los Angeles is 198 m² and they cost, on average, $727 per night. Moreover, these rentals are a great option for 7 it represents the average number of vacationers house rental can accommodate in.
[Solved] consider the reaction mg(s)+2hcl(aq)→mgcl2(aq)+h2(g) the total... Course Hero
Hasil dari reaksi propuna dengan HCl adalah senyawa 2-kloropropena. Penjelasan : Reaksi propuna dengan HCl merupakan reaksi adisi alkuna yang akan menghasilkan senyawa alkena bersubstituen halida. Senyawa propuna merupakan alkuna asimetris atau senyawa alkuna yang ikatan rangkapnya terletak di antara atom C yang mengikat substituen berbeda.

Propane Torch Rimfire Central Firearm Forum
The balanced reduction half reaction is as follows: 2H+ + 2e− → H2 (11.5.5) (11.5.5) 2 H + + 2 e − → H 2. There are two hydrogen atoms on each side, and the two electrons written as reactants serve to neutralize the 2+ charge on the reactant hydrogen ions. Again, the overall charge on both sides is zero.

3 Economical Benefits of Propane Low Price Gas Bulverde NearSay
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: The chlorination of propane proceeds as a radical chain reaction. Sort the 7 reaction steps (you may need to scroll down to see them all) into categories of:
Propane Energy Education
Ca + 2HCl CaCl 2 + H 2 ↑ (ii) Action on oxides — Hydrochloric acid reacts with oxides to form salt and water only. CuO + 2HCl CuCl 2 + H 2 O (iii) With salts of weaker acids — Hydrochloric acid decomposes salts of weaker acids. Na 2 CO 3 + 2HCl 2NaCl + H 2 O + CO 2 ↑. Question 12. MnO 2, PbO 2 and red lead react with conc. HCl acid.
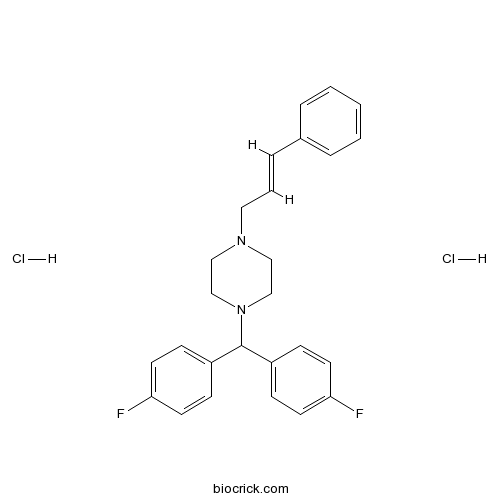
Flunarizine 2HCl Flunarizine 2HCl 30484776 天然产物(标准品)百奥克睿官网
Example 7.4.6 7.4. 6: Predicting the outcome of a neutralization reaction. Write the balanced chemical equation for the neutralization of HCl with Mg (OH) 2. Hint: neutralization reactions are a specialized type of double replacement reaction. 2HCl + Mg (OH) 2 → Mg (Cl) 2 + 2H 2 O. Since HCl is a strong acid and Mg (OH) 2 is a strong base.

Lpg Propane Clearance Buy, Save 58 jlcatj.gob.mx
3. When a Cl· atom is knocking a propane molecule, it may touch it at the end of the molecule, where there are 6 H atoms. Or it may touch it by the middle, where there are are only 2 H atoms. The probability that the collision happens with the end carbon is at least 3 times higher than the other possibility.

3,3',5,5'四甲基联苯胺盐酸盐(TMB.2HCl) 瑞思试剂
Jika hanya isomer struktur saja yang dihitung, maka heksena memiliki jumlah paling banyak yaitu 5 struktur, sedangkan heksana dan heksuna masing-masing memiliki 3 struktur. Soal No.30. Contoh soal reaksi senyawa hidrokarbon. Tuliskan persamaan reaksinya dan berikan nama senyawa yang dihasilkan. 2-metil-2-butena + H 2.
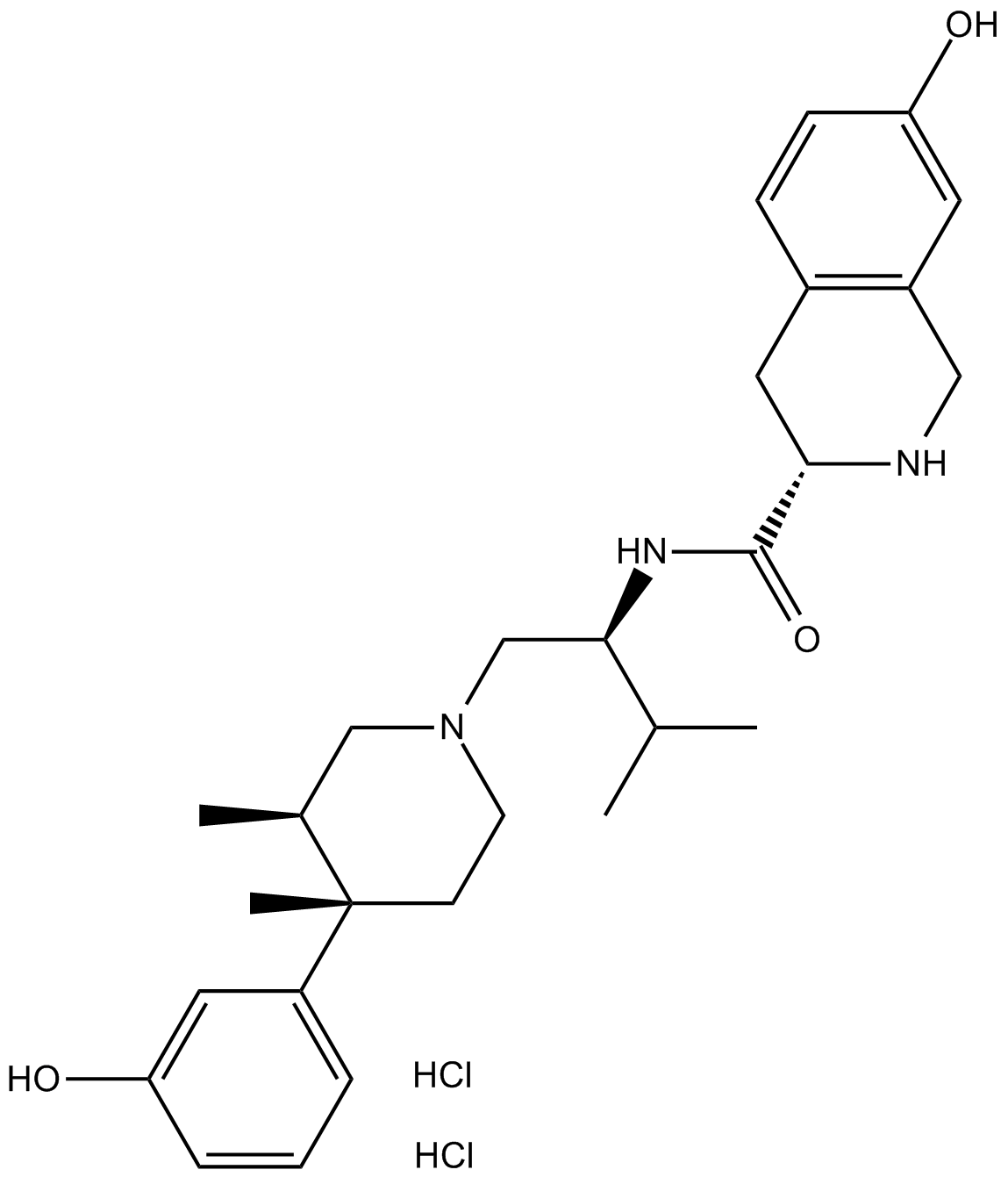
APExBIO JDTic 2HClκopioid receptor antagonistCAS 785835792
Chemistry questions and answers. The chlorination of propane proceeds as a radical chain reaction. hy 2 CH CH CH, + 2C1 CH CH CH CI+CH CHCICH, + 2 HCI Sort the 7 reaction steps (you may need to scroll down to see them all) into categories of initation, propagation and termination. Initiation Propagation Termination CH3CH2CH2-H - CH3CH2CH2.

20 lb Propane Tank Beverage Elements
Exercise 3.1 3. 1. Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned spacecraft and submarines. Write an equation for the reaction that involves 2 mol of LiOH per 1 mol of CO 2. (Hint: Water is one of the products.) Answer. Exercise 3.2 3. 2.