
Pengertian Dan Rumus Molaritas Dan Contoh Soal Molaritas Lengkap Riset Riset
Note that the solution density is assumed to be 1.00 g/mL. This is the common practice in ppm calculations. 2) Convert to ppm by multiplying numerator and denominator by 1000: 0.4008 g / 1000 g of solution times 1000/1000 = 400 g / 1,000,000 g of solution The answer is 400 ppm. Notice how the weight for calcium ion only is used.
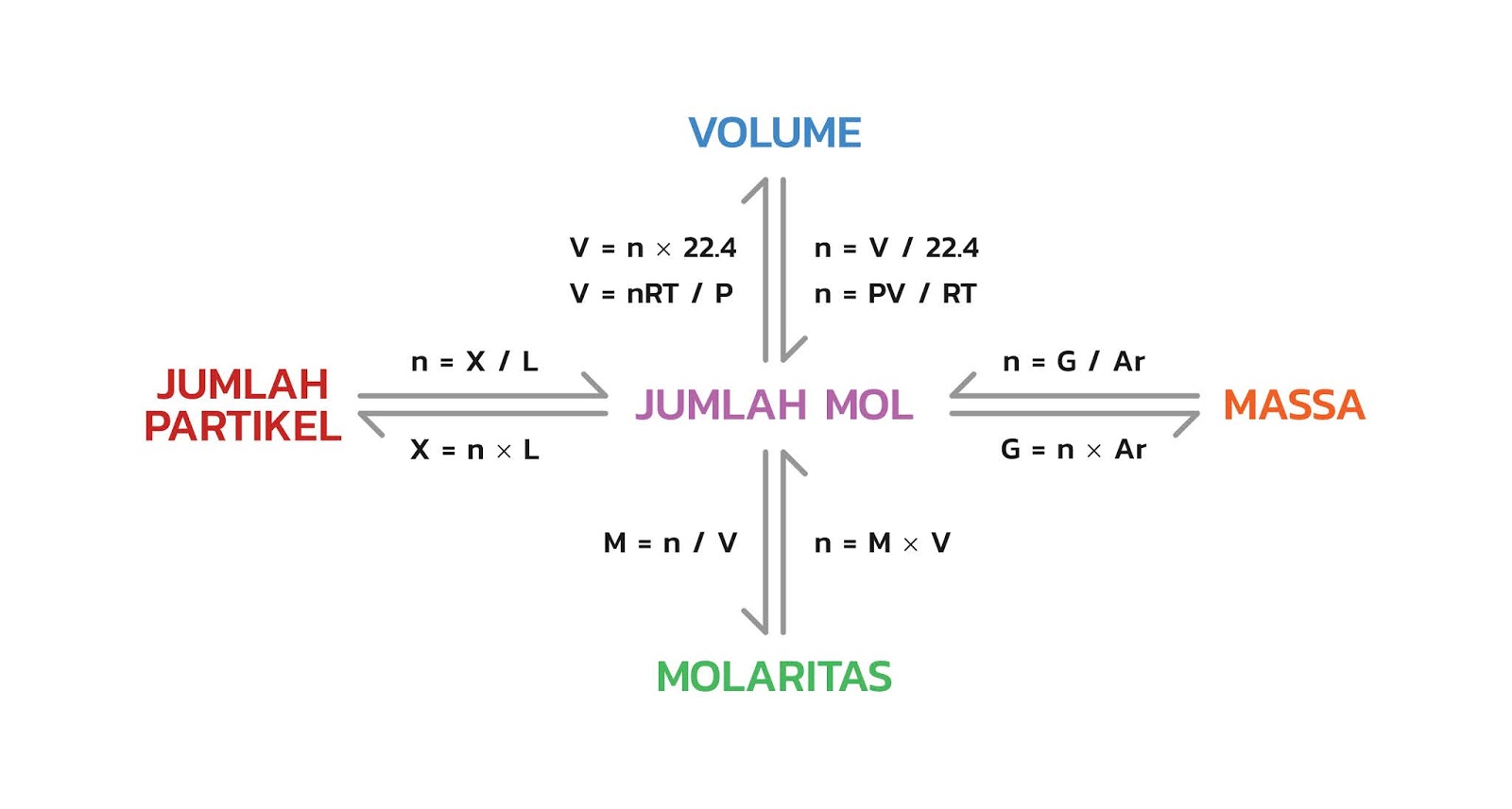
Hubungan Mol Dengan Jumlah Partikel Massa Volume Dan Molaritas Riset
Kalkulator konversi mol per liter (mol / L) ke miligram per liter (mg / L) ke ppm. Larutan air, pengonversi konsentrasi molar (molaritas) ke miligram per liter menjadi bagian per juta (ppm). Konsentrasi molar (molaritas): c (mol / L) =. perempuan jalang. Massa molar terlarut:

Soal 3. Konversi satuan konsentrasi ppm ke molaritas YouTube
Both ppm (parts per million) and molarity are measures of concentration. Did you know that ppm is used in different ways depending on the context? When dealing with dilute solutions, 1 ppm can be approximated as 1 mg 1\ \text{mg} 1 mg of substance per liter of water, or 1 mg / L 1\ \text{mg}/\text{L} 1 mg / L.. On the other hand, molarity is molar concentration, meaning that it tells you how.

Convertir de ppm a Molaridad y de Molaridad a ppm YouTube
1 ppm = 1 μg X / ( mL solution)x (1 L/1000 mL) 1 ppm = 1000 μg X / L solution. 1 ppm = 1 mg X/L solution. We know the molarity of the solution, which is in moles/L. We need to find mg/L. To do this, convert moles to mg. moles/L of Cu 2+ = 3 x 10 -4 M. From the periodic table, the atomic mass of Cu = 63.55 g/mol.

How to calculate formal concentration from ppm to molar in a dilution problem YouTube
Here are the steps to convert ppm to molarity. First, calculate the ppm of the solution. Second, Calculate the molecular weight (molar mass) of the substance for which you want to convert ppm to molarity. finally, put values into the formula and calculate the molarity.
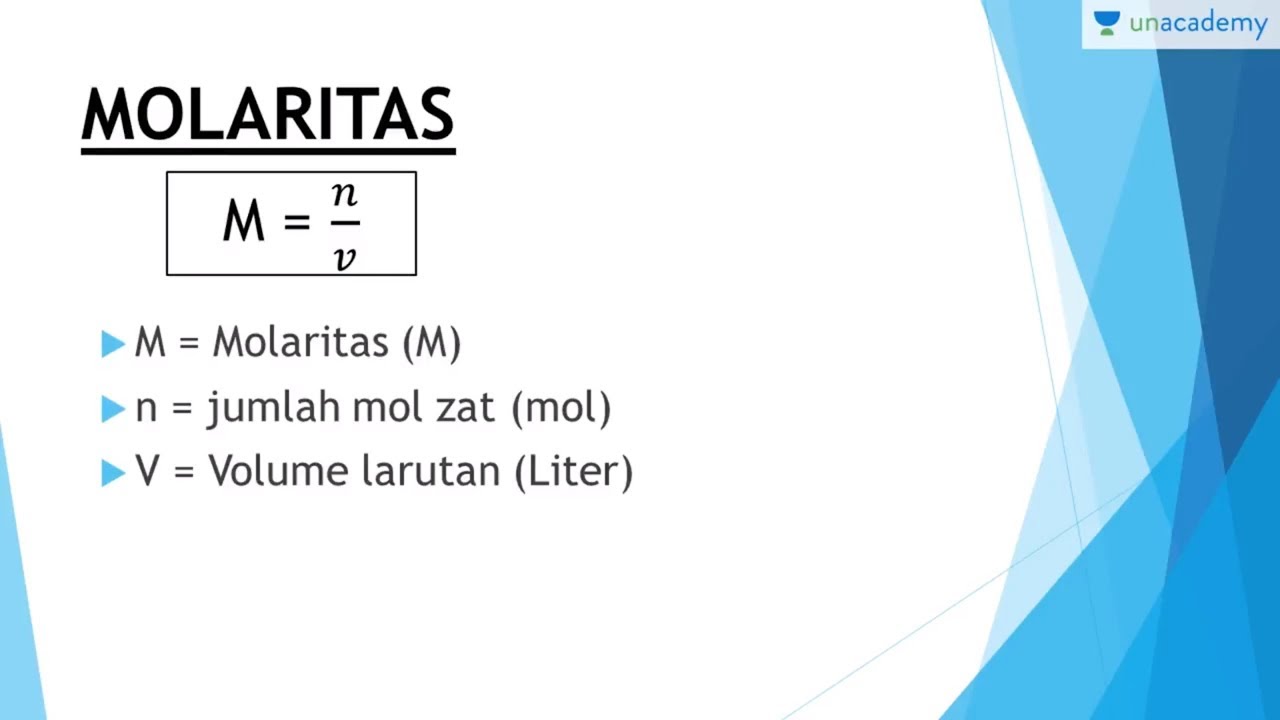
Konsep Mol Molaritas (Stoikiometri Kimia SBMPTN, SMA) YouTube
Ingat, rumus molaritas pencampuran adalah: Maka, konsentrasi larutan setelah dicampurkan adalah: M campuran = (100 x 0,1) + (150 x 0,2) / (100 + 150) = 40 / 250. = 0,16 M. Quipperian, itu dia pembahasan mengenai rumus molaritas beserta contoh soal dan pembahasannya. Agar semakin paham dan mahir dalam menggunakan rumus molaritas, cobalah untuk.

Perbedaan Molaritas Dan Molalitas Riset
Larutan air, pengonversi konsentrasi molar (molaritas) ke miligram per liter menjadi bagian per juta (ppm). Masukkan konsentrasi molar (molaritas): c (mol / L) = perempuan jalang : Masukkan massa molar terlarut:. Bagaimana cara mengubah ppm ke ppb. Bagian P dalam ppb sama dengan bagian P dalam ppm dikalikan 1000: P (ppb) = P (ppm) × 1000.
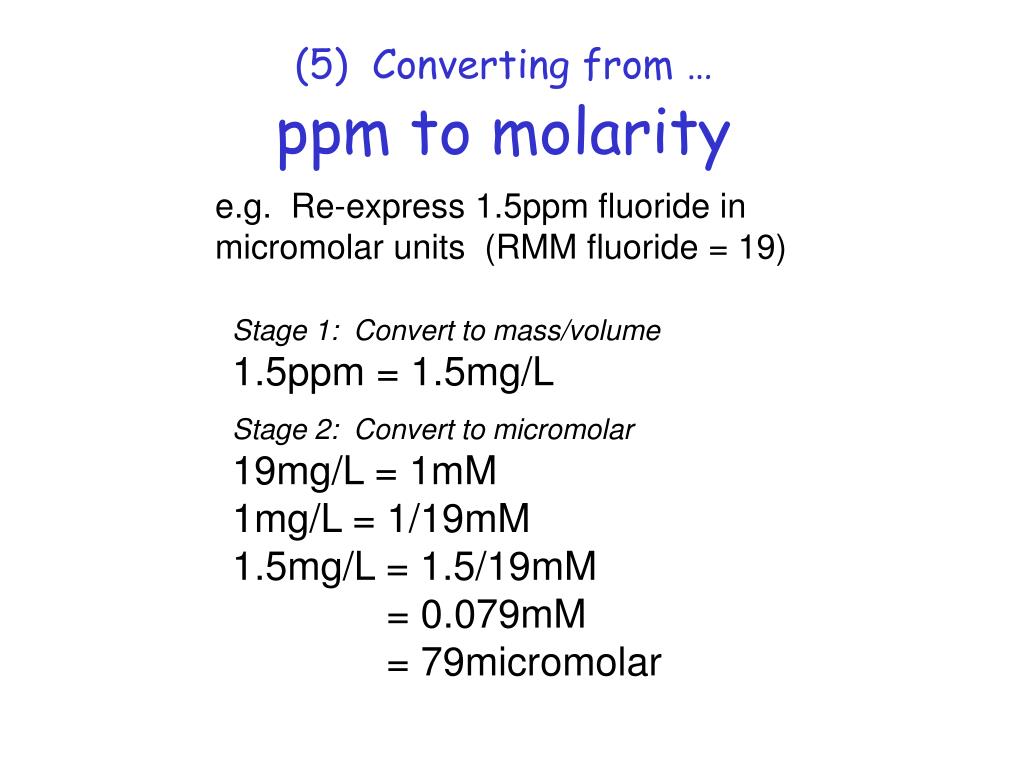
Molarity To Ppm Calculator slideshare
2. How many PPM is a mol? The PPM and Molarity table says that 1 M = 35,500 PPM. So, 35,500 ppm is equal to 1 mol per liter. 3. What is PPM? PPM is parts per million is a measure of concentration. It can be represented by mg/L and can be used in different ways depending on the context. PPM of a nutrient in the soil, that uses mg nutrient per kg.

CARA MUDAH MERUBAH PERSEN MENJADI MOLARITAS PADA SOAL PENGENCERAN 001 YouTube
Suatu larutan dengan konsentrasi 3 x 10 -4 M ion Cu 2+ setara dengan 19 ppm. ppm ke Contoh Konversi Molaritas . Anda juga dapat melakukan konversi unit dengan cara lain. Ingat, untuk larutan encer, Anda dapat menggunakan perkiraan bahwa 1 ppm adalah 1 mg / L. Gunakan massa atom dari tabel periodik untuk menemukan massa molar dari zat terlarut.

Pengertian Molaritas, Rumus dan Contohnya
To calculate molarity, we just need to insert these 2 numbers in the equation above like this (with the result): Molarity (500 PPM NaOH) = 500 PPM / (1000 × 40 g/mol) = 0.0125 mol/L. We can see that 500 PPM NaOh forms a 0.0125 M solution. That means that we have 0.0125 moles of NaOH in every liter of water solution.

PPM to Molarity Calculator Calculator Academy
To convert 1mg to ppm, you need to know the volume of the solution. Use the formula: ppm = (1 / molar mass) * 10^6 / volume of solution (in liters) What unit is the same as ppm? PPM (parts per million) is a unit of concentration that is equivalent to 1 milligram of solute per liter of solution.

Satuan Konsentrasi Ppm, , Molaritas dan contoh soal YouTube
Dalam bidang kimia ada beberapa satuan yang sering digunakan untuk menyatakan konsentrasi larutan ( banyaknya zat terlarut dalam sejumlah pelarut ). Beberapa satuan konsentrasi itu antara lain : mol, molalitas, molaritas, normalitas, ppm, persen massa, persen volum. Berikut akan saya jelaskan pengertian dan rumus dari satuan-satuan di atas : 1.

How to convert ppt to molarity of a water solution Analytical Chemistry YouTube
ppm ke Contoh Konversi Molaritas. Anda juga dapat melakukan konversi satuan dengan cara lain. Ingat, untuk larutan encer, Anda dapat menggunakan perkiraan bahwa 1 ppm adalah 1 mg/L. Gunakan massa atom dari tabel periodik untuk menemukan massa molar zat terlarut.
Molarity percent by mass ppm simple calculations YouTube
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright.

Molaritas Pengertian, Rumus, Pembuatan, Pengenceran Larutan Dan Contohnya Lengkap BprsKu.Co.Id
PPM to Molarity Formula. The following equation is used to convert PPM to molarity. M = PPM/ mm / 1000 M = PPM /mm/1000. Where M is the molarity. mm is the molar mass (g/mol) PPM is the parts. To calculate molarity from ppm, divide the PPM by the molar mas (g/mol), then divide the result by 1000.

Kimia 12 Molaritas Molalitas dan Fraksi Mol YouTube
Bagaimanakah caranya mengubah satuan konsentrasi larutan, dari persen massa menjad satuan molaritas?Pada proses konversi satuan ini selalu dibutuhkan data ma.