
Atomic Theory III, Video I Orbital Hybridization and Bonding sp3 orbitals YouTube
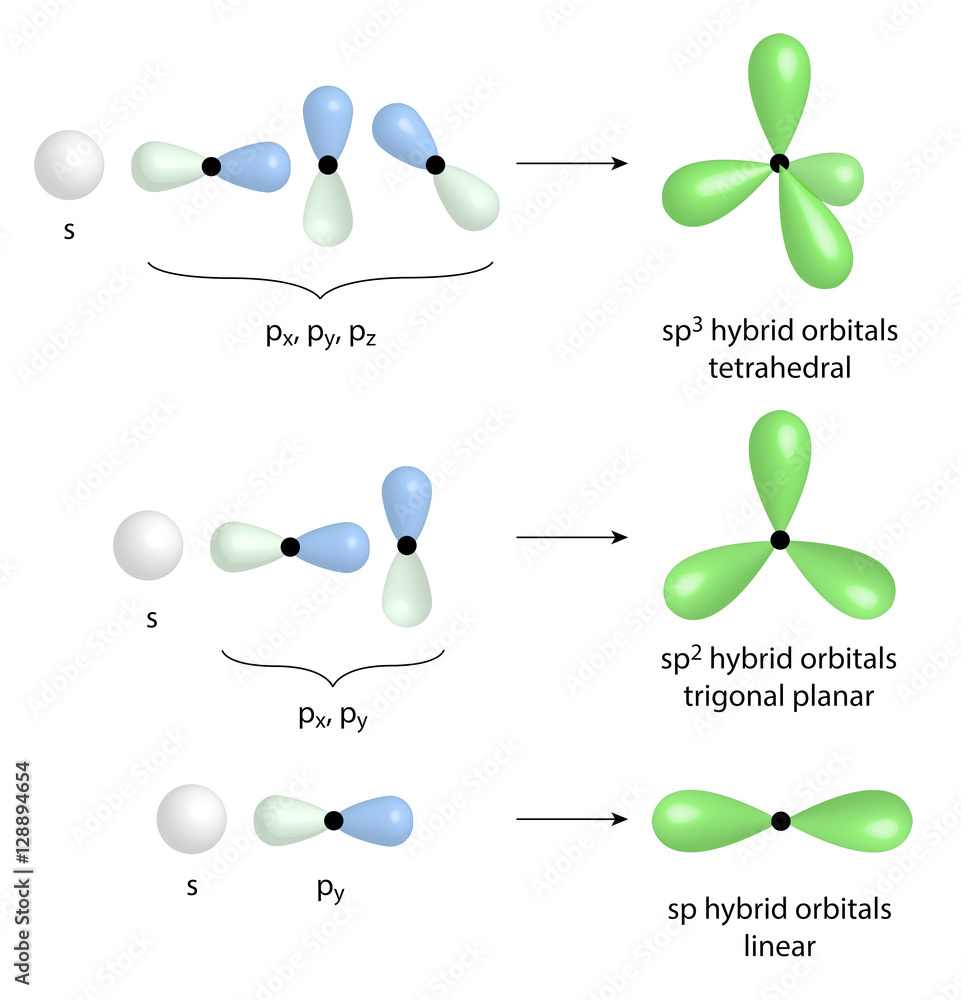
An sp2 hybrid orbital is composed of three orbitals, one s and two p, so the s character is 1/3 or 33.3% s while the p character is 2/3 or 66.7%. The shape is similar to an sp3 orbital, but the increased s character makes an sp2 orbital even smaller than an sp3 orbital. The same logic follows for sp orbitals having ½ s (50%) and ½ p character.
Valence Bond Theory and Hybrid Orbitals Introductory Chemistry, 1st Canadian Edition [Clone]
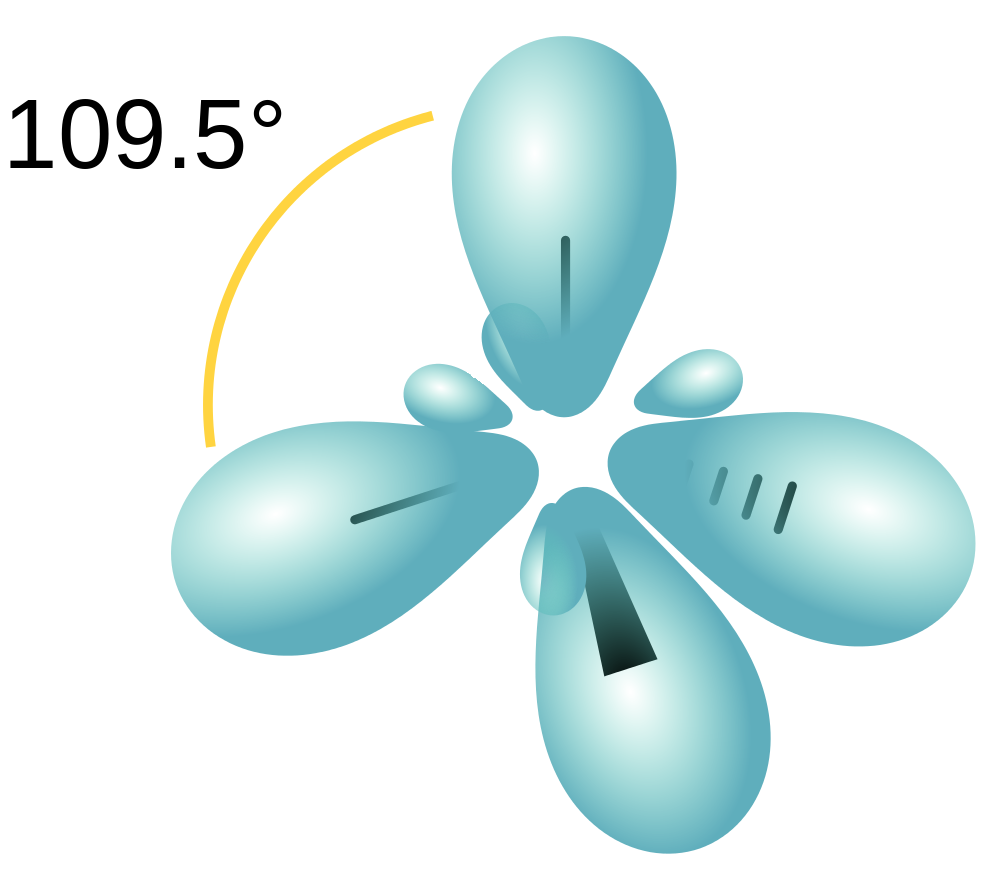
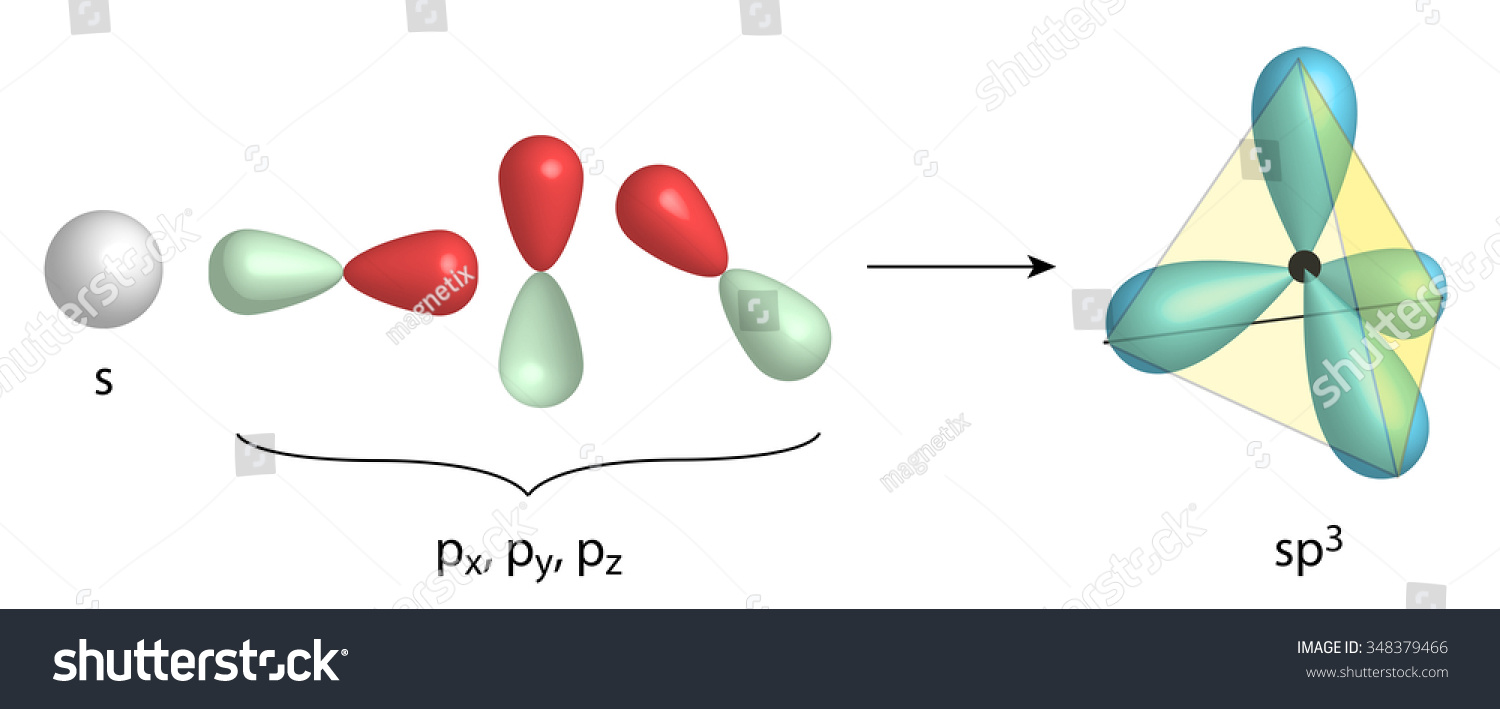
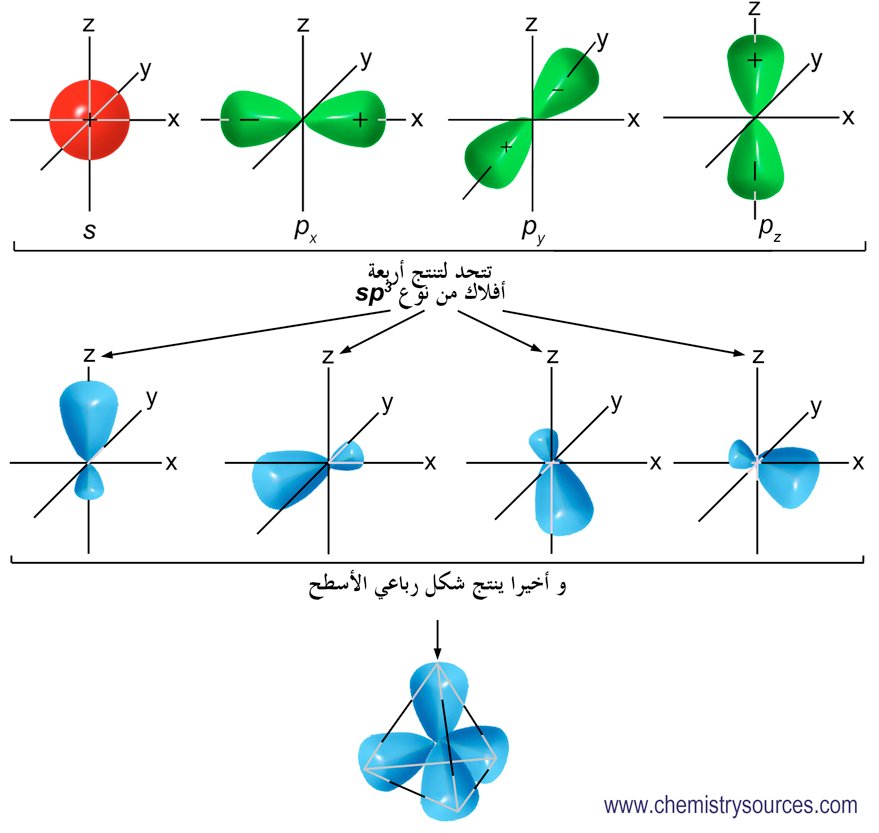
The hybridization of an s orbital (blue) and three p orbitals (red) produces four equivalent sp3 hybridized orbitals (yellow) oriented at 109.5° with respect to each other. A molecule of methane, CH 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. The carbon atom in methane exhibits sp3 hybridization.

Orbital HYBRIDIZATION Of The Central Atom / sp3, sp3d, sp3d2 (Just Considering Sigma Bonds
→ anggap ada 1s + 3p → orbital hibrida S adalah sp 3. Bagaimana menentukan orbital hibrida jika tidak diberikan gambar struktur molekulnya? Kembali seperti bahasan sebelumnya, misal PH 3 atau PCl 3 orbital hibrida P adalah sp 3, H dan Cl yang berikatan pada P tidak mungkin membentuk ikatan dobel atau tripel, semuanya membentuk ikatan singel.
Orbitales Híbridos Orbitales Híbridos.
The process of sp 3 hybridization is the mixing of an s orbital with a set of three p orbitals to form a set of four sp 3 hybrid orbitals. Each large lobe of the hybrid orbitals points to one corner of a tetrahedron. The four lobes of each of the sp 3 hybrid orbitals then overlap with the normal unhybridized 1s orbitals of each hydrogen atoms.

Sp3 hybrid orbital Chemistry Dictionary & Glossary
This organic chemistry video tutorial explains the hybridization of atomic orbitals. It discusses how to determine the number of sigma and pi bonds in a mol.
sp3 sp2 sp hybrid orbitals Stock Illustration Adobe Stock
Orbital hybridisation. In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which.

Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp, Sp2, Sp3 Membership YouTube
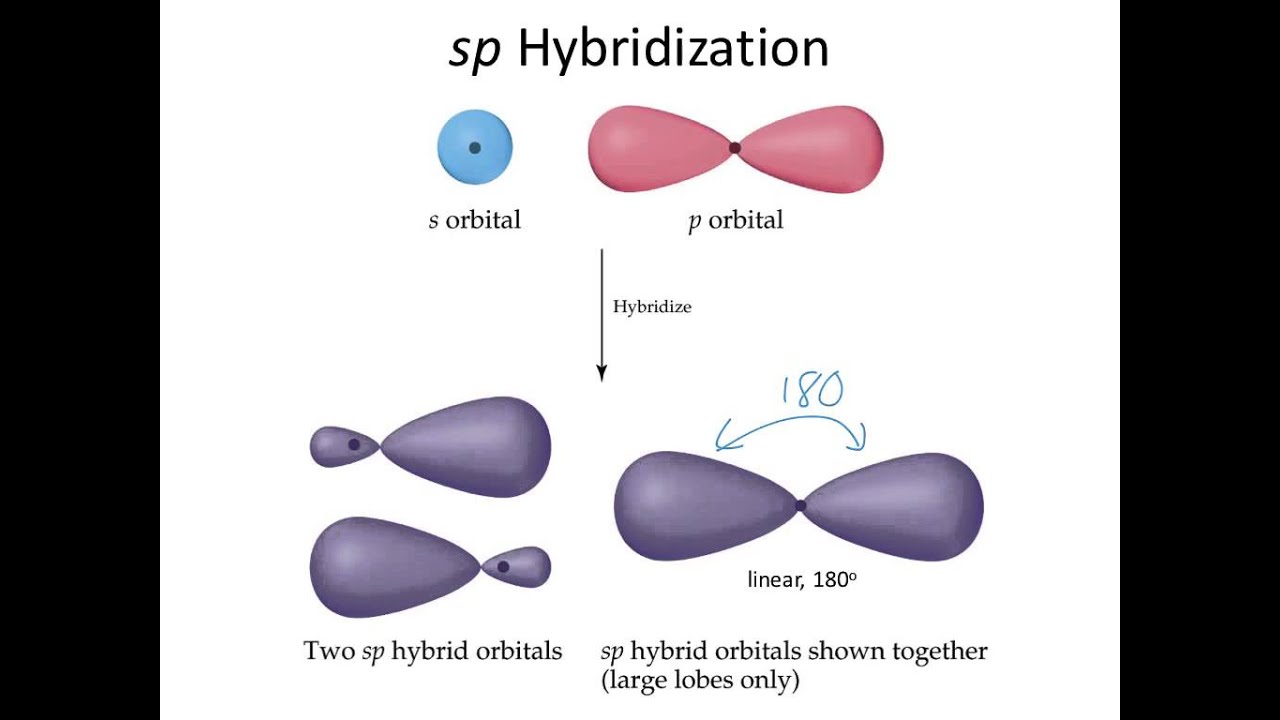
Orbital sp 2 memungkinkan pembentukan tiga ikatan σ, sedangkan orbital p murni ikatan π (ikatan rangkap dua atau rangkap tiga menyiratkan satu atau dua ikatan π). Misalnya,. Orbital hibrida dipisahkan oleh sudut 180º. Untuk alasan ini, atom-atom yang terhubung diatur dalam geometri molekul linier (B-A-B). Akhirnya, pada gambar di bawah.

Introduction to Electron Orbital Hybridization (sp3 sp2 & sp Made Super Simple!) Organic
An sp3 orbital looks something like this. This is a hybridized sp3 orbital. Hybrid just means a combination of two things. A hybrid car is a combination of gas and electric. A hybridized orbital is a combination of s and p. Hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything.

Orbitales híbridos Recursos educativos digitales
Introduction. The term "sp 3 hybridization" refers to the mixing character of one 2s-orbital and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be sp 3 hybridized, it must have an s orbital and three p orbitals.. From wave function to the visual representation: Four equivalent sp3 hybrid orbitals, resulting from the combination of one.

AP Chemistry Molecular Geometry Part 9 sp3 Hybrid Orbitals YouTube
The nitrogen atom is sp 3 hybridized with one hybrid orbital occupied by the lone pair. The molecular structure of water is consistent with a tetrahedral arrangement of two lone pairs and two bonding pairs of electrons. Thus we say that the oxygen atom is sp 3 hybridized, with two of the hybrid orbitals occupied by lone pairs and two by bonding.
Sp3 Hybrid Orbitals From S And POrbitals Stock vektorkép 348379466 Shutterstock
Lung Mei, Sai Kung District. Coordinates: 22.393785°N 114.269061°E. Lung Mei ( Chinese: 龍尾) is a village in Sai Kung District, Hong Kong .
Orbital Hybridization sp3 sp2 sp sigma YouTube
The sp3 s p 3 hybrids are all equivalent to one another. Spatially, the hybrid orbitals point towards the four corners of a tetrahedron (see figure below). Figure 9.22.5 9.22. 5: The process of sp3 s p 3 hybridization is the mixing of an s s orbital with a set of three p p orbitals to form a set of four sp3 s p 3 hybrid orbitals.
:max_bytes(150000):strip_icc()/hybrid-orbital-38d0c0f547ef46268127f68ce20714fa.jpg)
Apa itu Orbit Hibrida dalam Kimia?
Wednesday 20 December 2023. Sai Kung is one of the best scenic escapes in Hong Kong. The district attracts nature adventurers and water-sport junkies alike who come for its idyllic beaches and a.

SP3 Hybridization Pi bond, Mcat, Line chart
To describe the five bonding orbitals in a trigonal bipyramidal arrangement, we must use five of the valence shell atomic orbitals (the s orbital, the three p orbitals, and one of the d orbitals), which gives five sp 3 d hybrid orbitals. With an octahedral arrangement of six hybrid orbitals, we must use six valence shell atomic orbitals (the s orbital, the three p orbitals, and two of the d.
What are sp3 orbitals? SolvedLib
Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron.

Orbital Hibrida sp, sp2, dan sp3 YouTube
1.7 sp3 Hybrid Orbitals and the Structure of Ethane. The same kind of orbital hybridization that accounts for the methane structure also accounts for the bonding together of carbon atoms into chains and rings to make possible many millions of organic compounds. Ethane, C 2 H 6, is the simplest molecule containing a carbon-carbon bond.