
Ondansetron Hydrochloride Injection at Best Price in India
For IV infusion, dilute ondansetron hydrochloride injection in 50 mL of 5% dextrose injection or 0.9% sodium chloride injection. For IV injection, no dilution required. Rate of Administration. IV infusion: Infuse over 15 minutes. IV injection: Inject over a period of ≥30 seconds, preferably over 2-5 minutes. IM Administration

2 mg Ondansetron Hydrochloride Injection, Rs 11.4 /strip Estrellas Life Sciences Private Limited
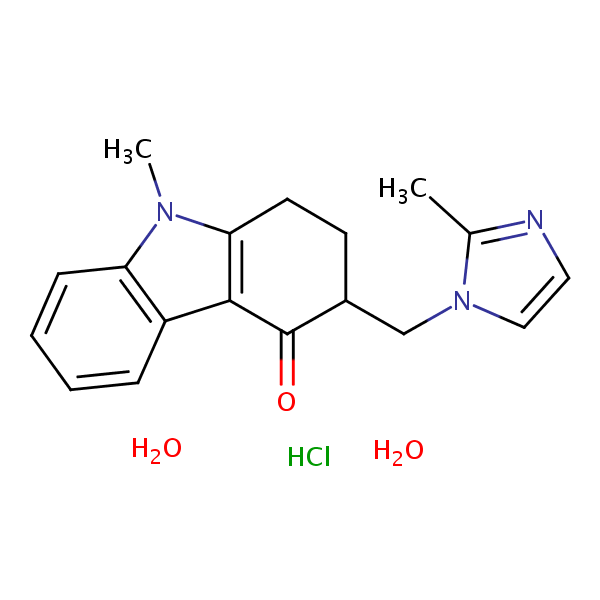
The molecular formula is C18H19N3O•HCl•2H2O,. Ondansetron tablets, USP for oral administration contain ondansetron hydrochloride USP (dihydrate) equivalent to 4 mg or 8 mg or 24 mg of ondansetron. Each film-coated tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch (maize.

Pill Identification Images of Ondansetron Hydrochloride Size, Shape, Imprints and Color
The molecular formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.9. Ondansetron HCl dihydrate is a white to off-white powder that is soluble in water and normal saline. Each 4 mg ondansetron tablet, USP for oral administration contains ondansetron hydrochloride dihydrate equivalent to 4 mg of ondansetron.

Ondansetron HCL Injection (2mg/ml) 20ml Bottle 1Family 1Health Pharmacy
The molecular formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.86. Ondansetron HCl dihydrate USP is a white to off-white powder that is soluble in methanol, sparingly soluble in purified water and in alcohol, and slightly soluble in isopropyl alcohol, in dichloromethane, very slightly soluble in acetone, in chloroform.
Ondansetron hydrochloride dihydrate SIELC
1.5 Appearance. Ondansetron hydrochloride is obtained as a white or off-white powder.. 1.6 Uses and Applications. Ondansetron hydrochloride is the soluble form of ondansetron, a tetrahydrocarbazolone derivative with an imidazolylmethyyl group. It is the first of a class of selective serotonin 5-HT3 receptor antagonists indicated for the prevention of nausea and vomiting associated with initial.

Ondansetron Hydrochloride Oral Solution IP, for Personal, Packaging Size 30 Ml, Rs 35 /bottle
Ondansetron hydrochloride is a member of carbazoles. ChEBI. Ondansetron Hydrochloride is the hydrochloride salt of the racemic form of ondansetron, a carbazole derivative and a selective, competitive serotonin 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist with antiemetic activity.

Datrone Ondansetron Hydrochloride Tablets IP, Packaging Type Strip, 4 Mg, Rs 490 /box ID
To prevent nausea and vomiting associated with cancer chemotherapy the recommended adult oral dosage of ondansetron (ZOFRAN®) is a single 24-mg tablet administered 30 minutes before the start of single-day highly emetogenic chemotherapy; for moderately emetogenic cancer chemotherapy the recommended adult oral dosage is one 8-mg ondansetron (ZOFRAN®) tablet given twice a day (every 12 hours.

Daily Medication Pearl Ondansetron Hydrochloride (Zofran)
Ondansetron Tablets are a product of USP, a scientific organization that sets standards for the quality and purity of medicines. Ondansetron is a medication used to prevent nausea and vomiting caused by chemotherapy, radiation, or surgery. Learn more about the specifications, ingredients, and uses of Ondansetron Tablets from USP.

Ondansetron Hydrochloride Oral Solution, 2 Mg, Packaging Size 30 Ml, Rs 37 /bottle ID
Find patient medical information for ondansetron HCl oral on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.

Pill Identifier Ondansetron Hydrochloride NDC 55111154
C18H20ClN3O.2H2O; GR 38032 HCl; Ondansetron hydrochloride, 98%; CHEMBL1201111; Ondansetron hydrochloride- Bio-X; MKBLHFILKIKSQM-UHFFFAOYSA-N; DTXSID701027913;. Ondansetron hydrochloride is also being studied in the treatment of other conditions. NCI Cancer Drugs. 6.6 Clinical Trials. 6.6.1 ClinicalTrials.gov.
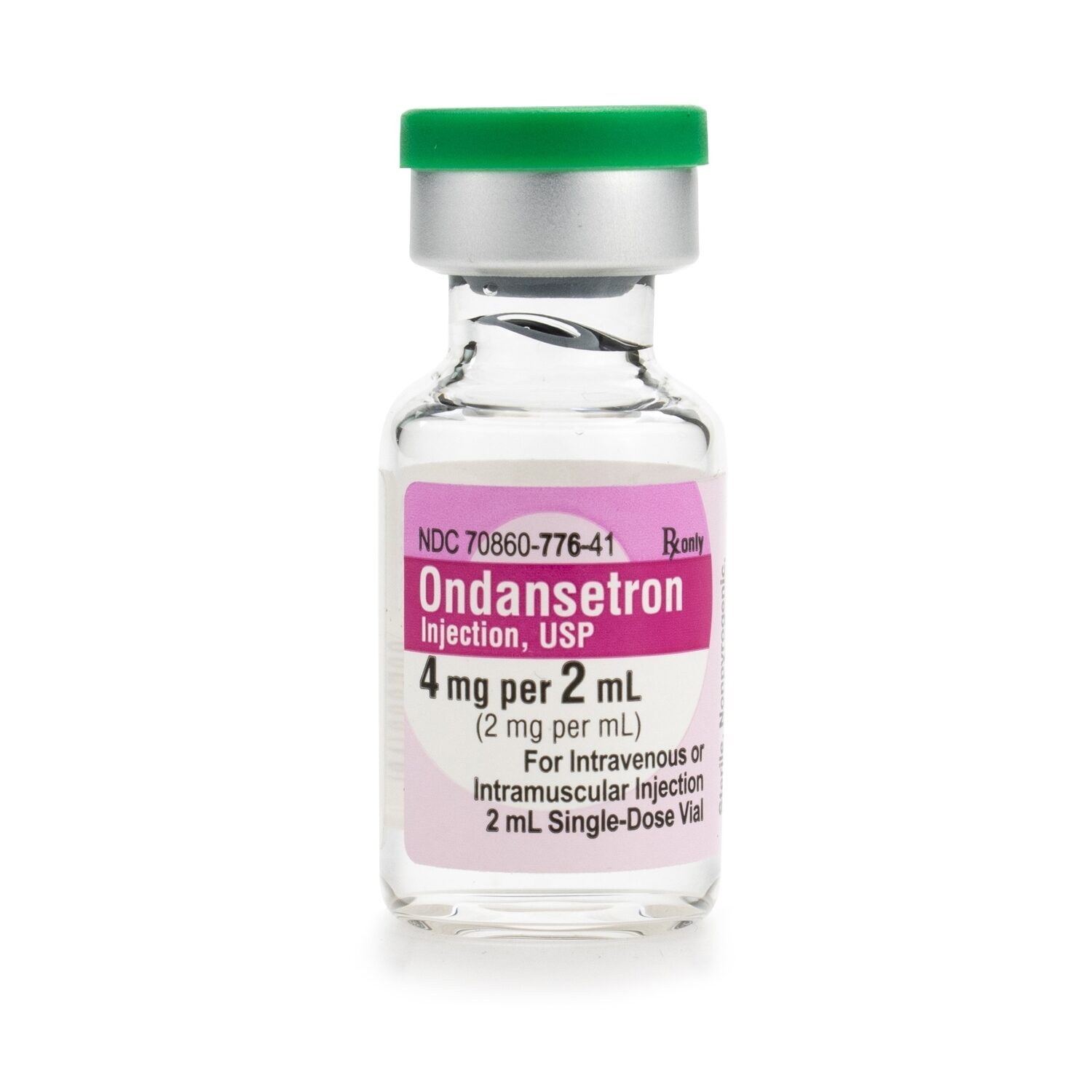
Ondansetron 2mg/mL SDV 2mL/Vial McGuff Medical Products
Buy Ondansetron hydrochloride USP compendial standard (CAS 103639-04-9) to determine strength, quality, purity and identity in your USP-NF monograph tests and assays.

Ondansetron Hydrochloride Oral Solution IP, 2mg, Rs 12.80 /pack ID 22580267591
Name. Ondansetron hydrochloride dihydrate. Drug Entry. Ondansetron. A competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties. Having been developed in the 1980s by GlaxoSmithKline.
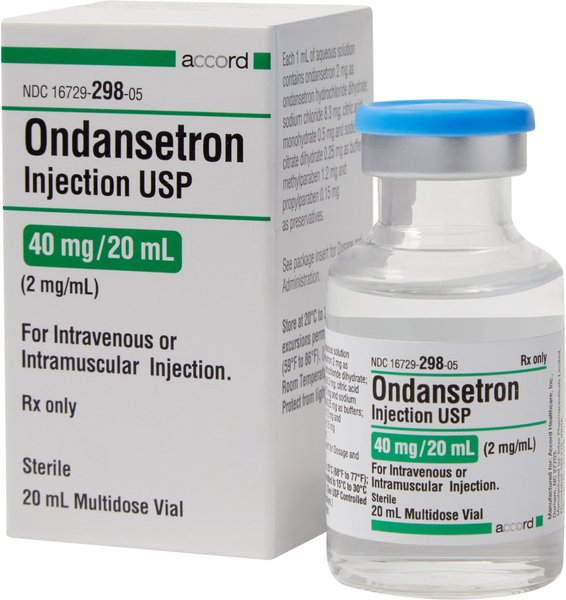
ONDANSETRON HCL (Generic) Injection, 2mg/mL, 20 ml
Ondansetron is a white to off-white powder that is sparingly soluble in water. Each 1 mL of the preservative-free aqueous solution in the 2-mL single dose vial contains 2 mg of ondansetron as the hydrochloride; 9 mg of sodium chloride; and 0.5 mg of citric acid monohydrate and 0.25 mg of sodium citrate dihydrate as buffers in water for injection.
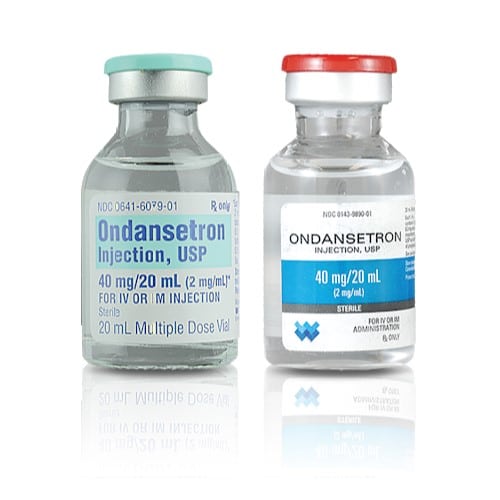
Ondansetron HCL Injection (2mg/ml) 20ml Bottle 1Family 1Health Pharmacy
Ondansetron is one of the medications most commonly used for the empiric treatment of nausea and vomiting. Ondansetron has excellent utility as an antiemetic drug, and it is effective against nausea and vomiting of various etiologies. Common uses of ondansetron include the prevention of chemotherapy-induced and radiation-induced nausea and vomiting, the prevention of postoperative nausea and.

ONDANSETRON HCL 2H2O YARINDO 4 MG TABLET Kegunaan, Efek Samping, Dosis dan Aturan Pakai
ONDANSETRON SAFETY. There is disagreement in the literature regarding the teratogenic risk of ondansetron use during pregnancy, particularly with regard to cardiac defects and cleft palates. The current dispute arose largely because of the publication of two large, retrospective studies 9, 10 and one abstract, which reported results that.

ONDANSETRON HYDROCHLORIDE USP DIHYDRATE PCCA
The molecular formula is C18H19N3O•HCl•2H2O,. Ondansetron tablets, USP for oral administration contain ondansetron hydrochloride USP (dihydrate) equivalent to 4 mg or 8 mg or 24 mg of ondansetron. Each film-coated tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch (maize.