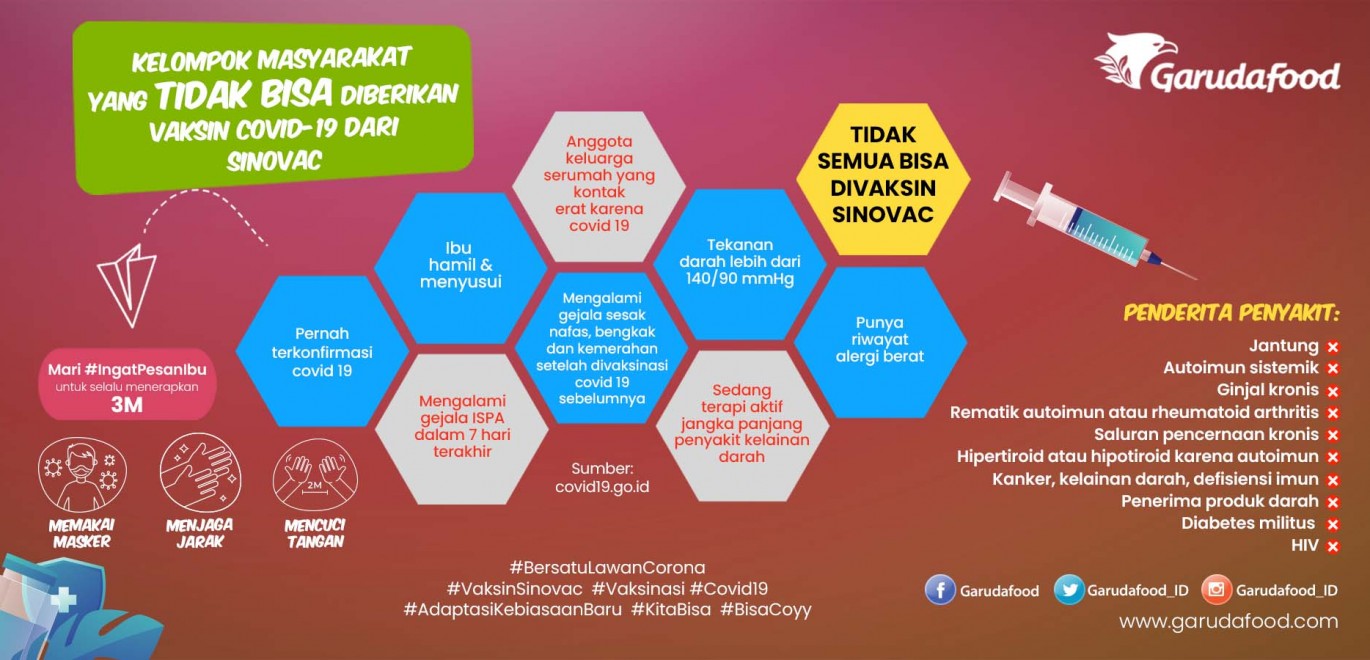
Tidak Bisa Diberikan Vaksin COVID19 dari Sinovac
Clinical symptom. Symptom A (for at least 2 days): Fever (Axillary temperature ≥37.5oC), chills, sore throat, fatigue, nasal congestion or runny nose, muscle pain, headache, nausea or vomiting, diarrhoea. Symptom B: Cough (for at least 2 days), loss of smell or taste (for at least 2 days), shortness of breath or difficulty breathing.
Sinovac Covid19 vaccine prevents 67 symptomatic infections in study
CoronaVac. CoronaVac, also known as the Sinovac COVID-19 vaccine, [3] is a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. [4] [5] It was phase III clinically trialled in Brazil, [6] Chile, [7] Indonesia, [8] the Philippines, [9] and Turkey [10] and relies on traditional technology similar to other.
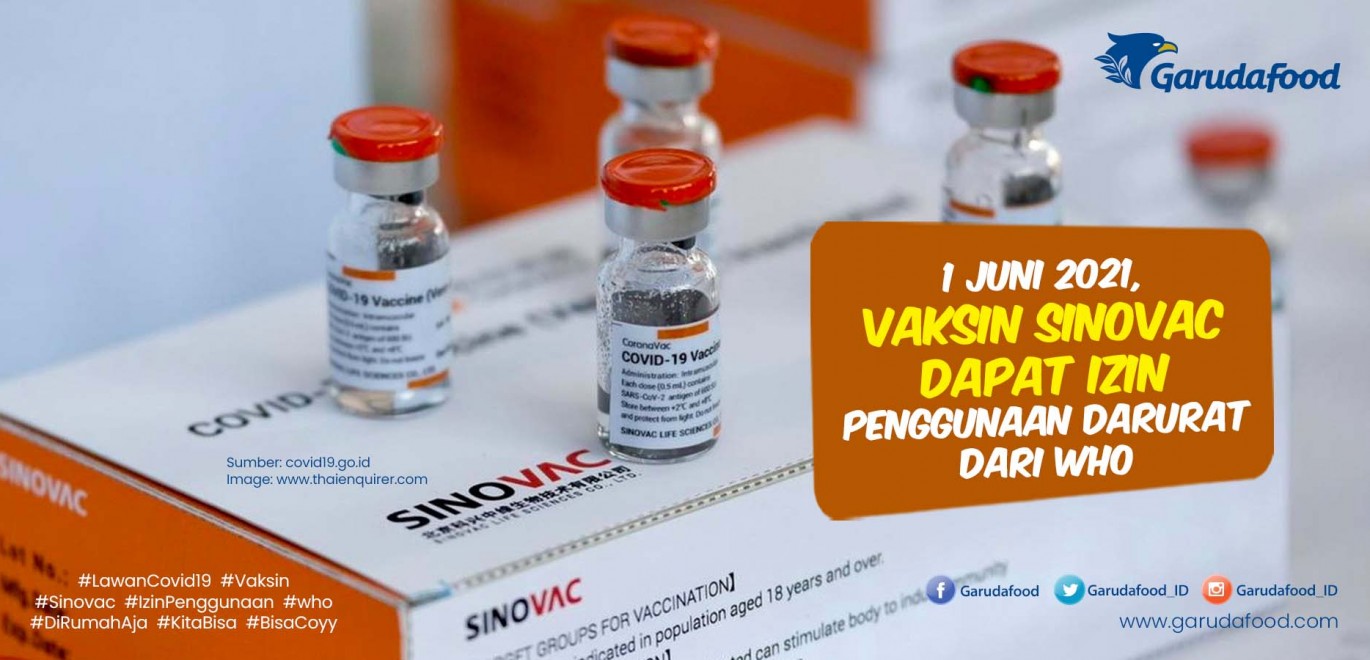
Vaksin Sinovac Dapat Izin Penggunaan Darurat dari WHO
Sering Disebut Terkait Obat dan Vaksin. Nomor batch adalah istilah lain untuk kode produksi, atau disebut juga nomor bets. Istilah ini kerap disebut oleh Badan Pengawas Obat dan Makanan (BPOM), termasuk dalam kaitannya dengan obat dan vaksin. Pedoman Cara Pembuatan Obat yang Baik (CPOB) menyebut, batch atau bets adalah sejumlah obat yang.
Vaksin Covid19 Bio Farma Aman Diberikan Kepada Anak Usia 6 11 Tahun
Dalam aturan terbaru ini vaksin COVID-19 merek Sinovac, AstraZeneca, Pfizer, dan Novavax tetap tidak dapat dipergunakan untuk Vaksinasi Gotong Royong. Ketentuan ini tertuang dalam Peraturan Menteri Kesehatan Nomor 18 Tahun 2021 yang disahkan oleh Menteri Kesehatan pada 28 Mei 2021, menggantikan Peraturan Menteri Kesehatan yang sebelumnya Nomor.

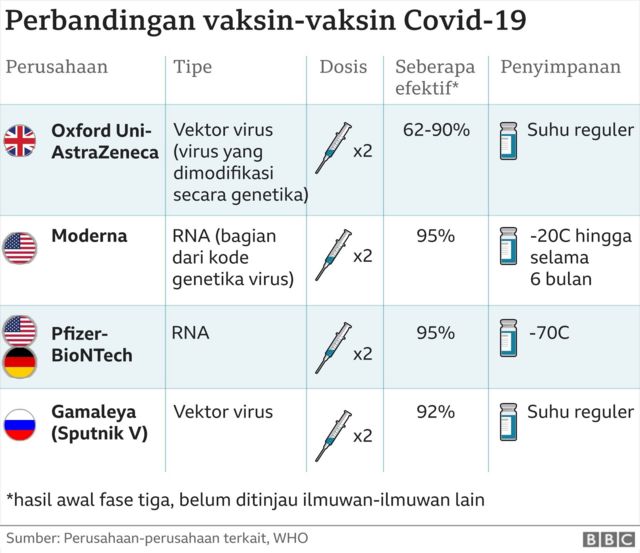
Vaksin Covid Apa perbedaan vaksin China, Sinovac dan Sinopharm serta merekmerek lain? BBC
Nomor batch vaksin Sinovac dapat ditemukan di sertifikat vaksin COVID-19, baik untuk dosis pertama maupun dosis kedua. Adapun untuk mengeceknya, ada beberapa hal yang harus dilakukan terlebih.

How the Sinovac Covid19 Vaccine Works The New York Times
Sinovac's commercialized vaccines include CoronaVac (COVID-19 vaccine), Inlive (Enterovirus 71 vaccine), Anflu (influenza vaccine), Healive (hepatitis A vaccine), varicella vaccine and mumps vaccine. COVID-19 vaccine development. CoronaVac is an.
:strip_icc():format(webp)/article/YkohzkJQClQN9UjzmCPPT/original/063035900_1611913523-Medfact-Vaksin-Sinovac-Mengandung-Chip-Pemantau-Ini-Faktanya-by-ANTARA-FOTO-Ardiansyah.jpg)
BPOM Setujui Vaksin Sinovac untuk Lansia, Ini Syaratnya KlikDokter
Manufacturer: Sinovac Life Sciences Co., Ltd. The COVID-19 Vaccine (Vero Cell) Inactivated, CoronaVac® is an inactivated vaccine against coronavirus disease 2019 (COVID-19) which stimulates the body's immune system without risk of causing a disease. Once the inactivated vaccine gets presented to the body's immune system, the production of.
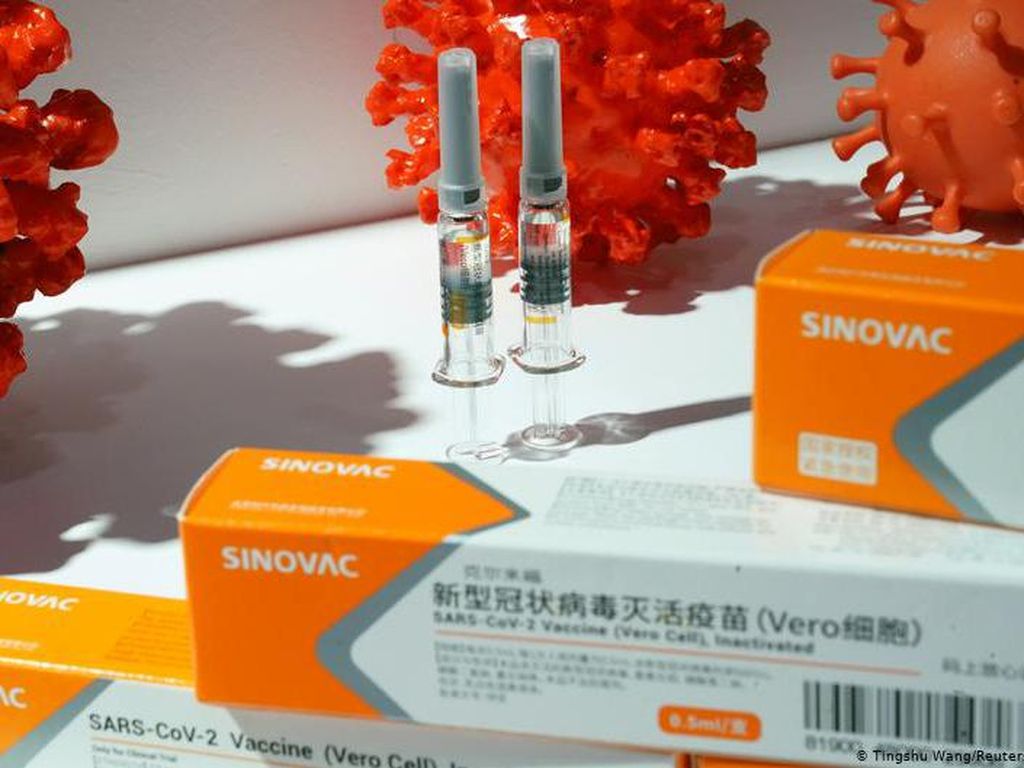
Berita dan Informasi Efektivitas vaksin sinovac Terkini dan Terbaru Hari ini
Two doses should be administered for primary. immunization. The second dose is preferably given 14-28 days after the first dose. CoronaVac should be administered by intramuscular injection in the deltoid region of the upper arm. It has not been determined whether this product requires booster immunization.

Jualan vaksin Sinovac lonjak pendapatan Pharmaniaga KLSE Screener
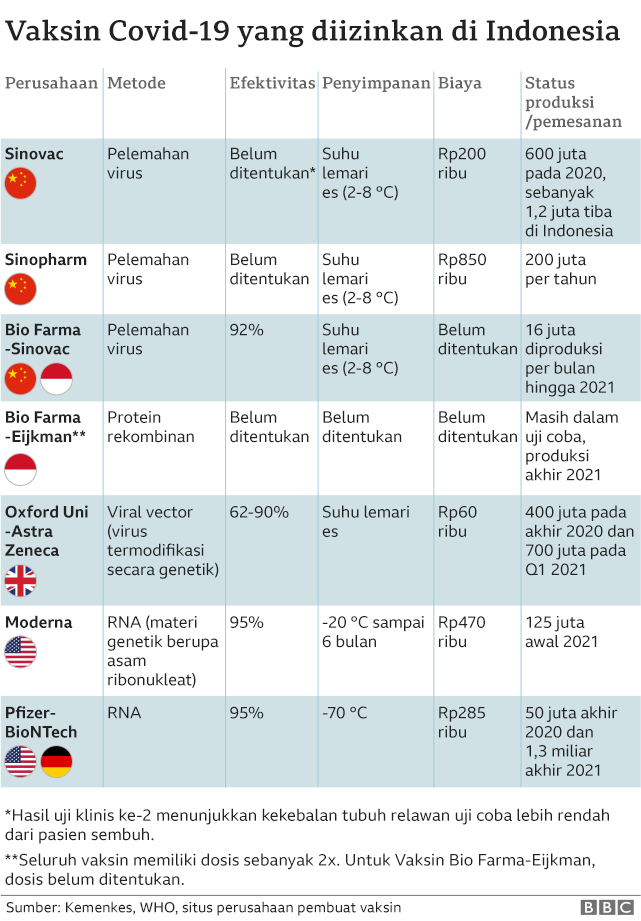
Dari 6 jenis vaksin Covid-19 yang sudah dan akan beredar di Indonesia, vaksin jenis Sinovac, AstraZeneca, Sinopharm, dan Moderna sudah mendapatkan Izin Penggunaan Darurat (EUA) dari Badan POM. Vaksin Sinovac EUA untuk Vaksin CoronaVac produksi Sinovac diterbitkan BPOM pada 11 Januari 2021. Efikasi vaksin ini, berdasarkan analisis uji klinik di.
Coronavirus How to encourage inoculation after a vaccine is developed
The Sinovac-CoronaVac COVID-19 vaccine uses an inactivated, no longer infectious form of the COVID-19 virus (SARS-CoV-2). The used COVID-19 virus is inactivated by a chemical that destroys the genetic material of the virus but leaves many proteins of the virus. The immune system reacts to these proteins and builds antibodies against the virus.
Vaksin Covid19 Keraguraguan tenaga kesehatan untuk disuntik vaksin Sinovac, 'kalau jadi bahan
The available data on the COVID-19 vaccine Sinopharm in pregnant women are insufficient to assess either vaccine efficacy or vaccine-associated risks in pregnancy. However, this vaccine is an inactivated vaccine with an adjuvant that is routinely used in many other vaccines with a documented good safety profile, including in pregnant women.

CoronaVac por que a Anvisa determinou a paralisação dos testes com a vacina da Sinovac/Butantan
The vaccine is safe and effective for all individuals aged 18 and above. In line with the WHO Prioritization Roadmap and the WHO Values Framework, older adults, health workers and immunocompromised persons should be prioritised. The Sinovac vaccine can be offered to people who have had COVID-19 in the past. But individuals may choose to delay.

Indonesia, which uses Sinovac vaccine is considering booster shot Ya Libnan
Kita selalu akan sampling setiap batch pembuatan. Vaksin yang datang dari Sinovac masuk ke sini, kita dampingi pada saat masuk, kemudian kita lakukan sampling, lot release untuk menguji aspek.
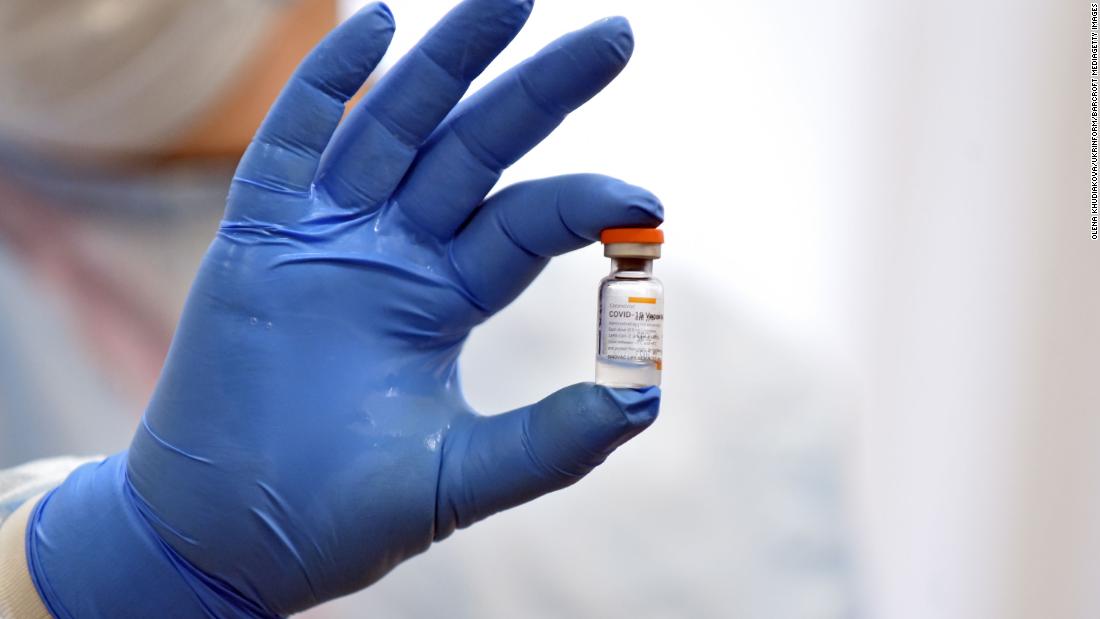
Sinovac WHO authorizes China's Sinovac Covid19 vaccine for emergency use CNN
A Vaccine Made From Coronaviruses. CoronaVac works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus. The antibodies attach to viral proteins, such as the so.

Vaksin Sinovac mengandung boraks dan "hanya untuk kelinci percobaan"? Cek faktanya! ANTARA News
Perbedaannya adalah di kemasannya. Diketahui memang ada 3 kemasan vaksin COVID-19 dari Sinovac yang berbeda-beda, yaitu: Sebelum pelaksanaan vaksinasi, Sinovac melakukan uji klinis vaksin ketiga.
Vaksin Covid19 mulai dikirim ke 34 provinsi di Indonesia BBC News Indonesia
According to the data, the most common side effect reported within 28 days of the second dose was injection-site pain (13-21%, depending on the dosing schedule). Injection site reactions are.