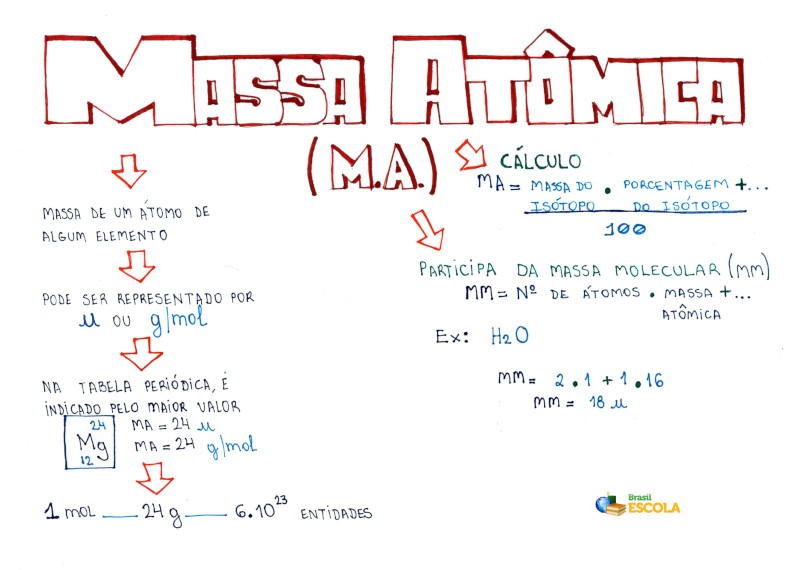
Cálculo da massa atômica Brasil Escola
Introduction. In chemistry, there are many different concepts of mass. It is often assumed that atomic mass is the mass of an atom indicated in unified atomic mass units (u).However, the book Quantities, Units and Symbols in Physical Chemistry published by the IUPAC clearly states: "Neither the name of the physical quantity, nor the symbol used to denote it, should imply a particular choice of.

Tabel Massa Atom Relatif Dan Bilanganoksidasi Unsur
Molar mass of P (Phosphorus) is 30.9737620 ± 0.0000020 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Phosphorus is a highly reactive, non-metallic element that exists in various forms, ranging from a white, waxy solid to a red or black powder.

Massa Atom Relatif dan Jarak Antar Atom berdasarkan Teori Fisika Materi Ahmad Dahlan
Tabel periodik modern, dalam tata letak 18 kolom. Tabel periodik, juga dikenal sebagai tabel periodik unsur (kimia), adalah tampilan tabular dari unsur-unsur kimia.Tabel ini banyak digunakan dalam kimia, fisika, dan ilmu-ilmu lainnya, dan umumnya dipandang sebagai ikon dari kimia.Tabel ini merupakan rumusan grafik dari hukum periodik, yang menyatakan bahwa sifat-sifat unsur kimia menunjukkan.

3 Modi per Calcolare la Massa Atomica wikiHow
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.

Nomor Atom dan Nomor Massa Kimia CoLearn
Sources: Green Book, 2 nd ed., p. 41 PAC, 1996, 68, 957. ( Glossary of terms in quantities and units in Clinical Chemistry (IUPAC-IFCC Recommendations 1996) ) on page 990 [ Terms ] [ Paper ] Citation: 'relative atomic mass' in IUPAC Compendium of Chemical Terminology , 3rd ed. International Union of Pure and Applied Chemistry; 2006.

Penjelasan Struktur Atom Proton, Neutron, Elektron dengan Contoh Soal
atomic mass, m. a. m. a. The commonly used unit is the. PAC, 1996, 68, 957. ( Glossary of terms in quantities and units in Clinical Chemistry (IUPAC-IFCC Recommendations 1996)) on page 962 [ Terms] [ Paper] Citation: 'atomic mass' in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006.
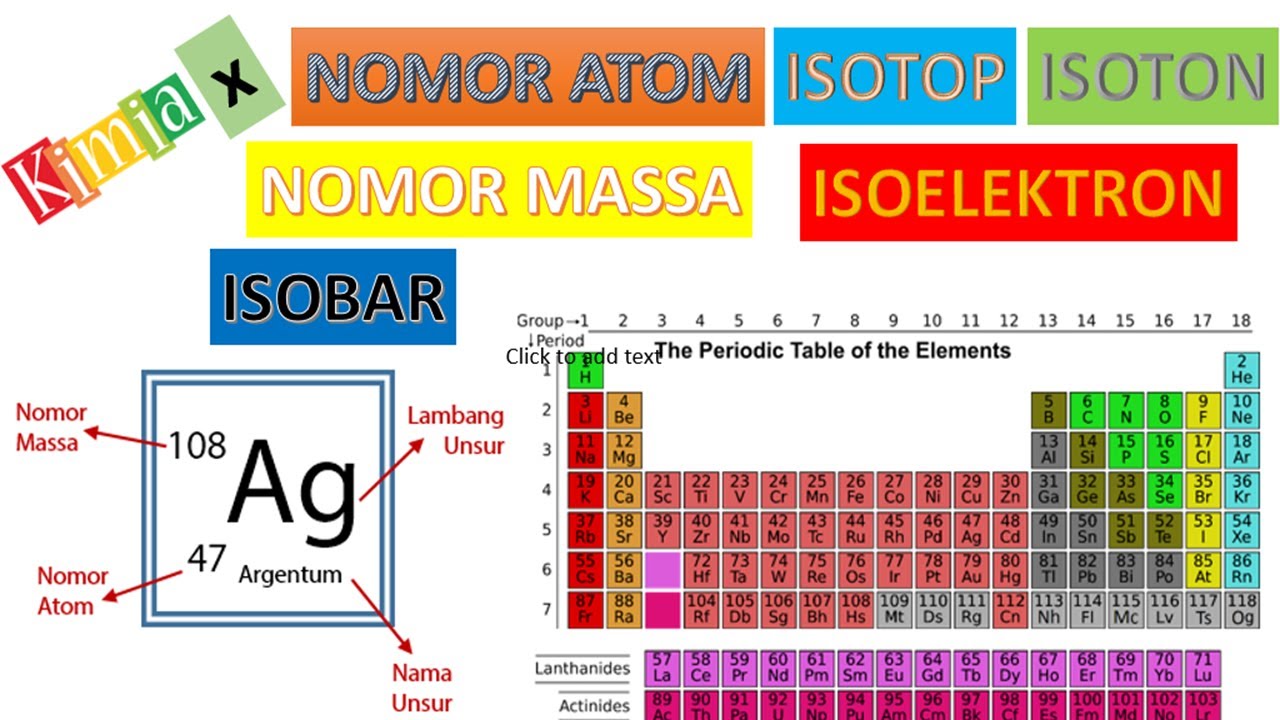
Kimia X Struktur Atom 2 Nomor Atom, Nomor Massa, dan Isotop YouTube
Karena atom C-12 ini digunakan sebagai standar, maka satu satuan massa atom didefinisikan sebagai suatu massa yang besarnya tepat sama dengan seperdua belas massa satu atom C-12. Nah, seperti sudah dibahas di infografik tadi, massa 1 atom hidrogen adalah 1,67 x 10 -27 kg , alias 1/12 dari massa atom C-12.

Massa Atom Relatif (Stoikiometri Kimia SBMPTN, SMA) YouTube
Untuk mencari massa atom relatif P , kita dapat menggunakan persamaan: Untuk mencari massa atom relatif P, kita dapat menggunakan persamaan: Perdalam pemahamanmu bersama Master Teacher di sesi Live Teaching, GRATIS! 77. 4.1 (10 rating) if. ifhal faizi. Jawaban tidak sesuaik. Iklan. Iklan. Klaim Gold gratis sekarang!.

MASSA ATOM RELATIF (Ar) DAN MASSA MOLEKUL RELATIF YouTube
Massa rata-rata satu atom P adalah 6,64 x 10-23 gram. massa satu atom karbon C-12 adalah 1,992 x 10-23 gram. Ditanyakan: Ar P =..? Jawaban: Soal UN Kimia 2019 No. 3. Perhatikan hubungan notasi unsur, konfigurasi elektron, dan letaknya dalam tabel periodik unsur berikut!

Tabel Massa Atom Relatif
Sulfur is a component of black gunpowder, and is used in the vulcanization of natural rubber and a fungicide. It is used to make sulfite paper and other papers, to fumigate, and to bleach dried fruits. It is also used extensively in making phosphatic fertilizers. Belerang - Sifat-sifat, sejarah, nama asli, fakta, kegunaan, isotop, konfigurasi.
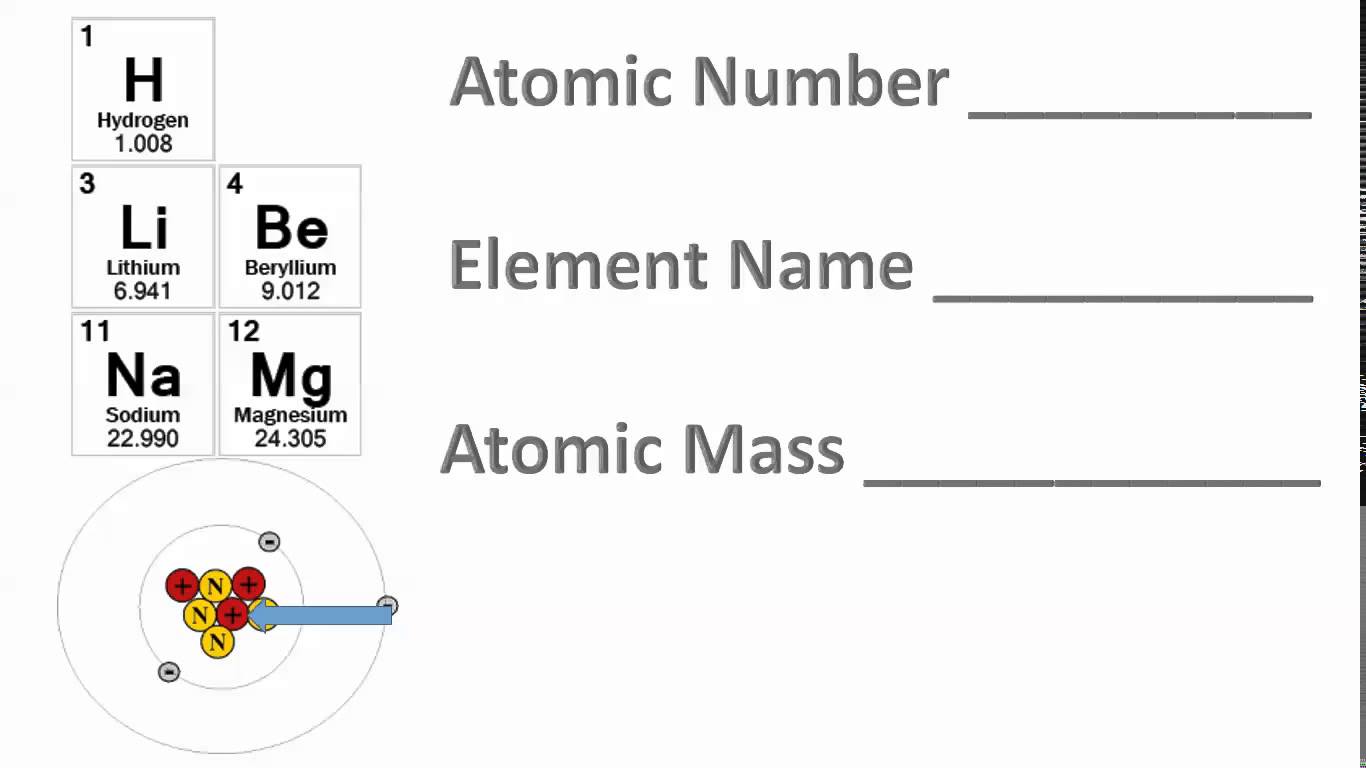
10 Contoh Soal dan Pembahasan Nomor Atom dan Nomor Massa Materi Kimia
Massa atom. 14,0067. Nomor massa. 14. Kategori. Nonlogam lainnya. Warna. Tak berwarna. Radioaktif. Tidak. Dari kata Latin nitrum, bahasa Yunani Nitron, soda asli; dan gen, membentuk. Struktur kristal. Segienam Sederhana. Sejarah. Nitrogen is considered to have been discovered by Scottish physician Daniel Rutherford in 1772, who called it.

Nomor Atom, Nomor Massa, dan Konfigurasi Elektron YouTube
The atomic mass (m a or m) is the mass of an atom.Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) - equivalently, unified atomic mass unit (u). 1 Da is defined as 1 ⁄ 12 of the mass of a free carbon-12 atom at rest in its ground state. The protons and neutrons of the nucleus account for nearly all of the total.
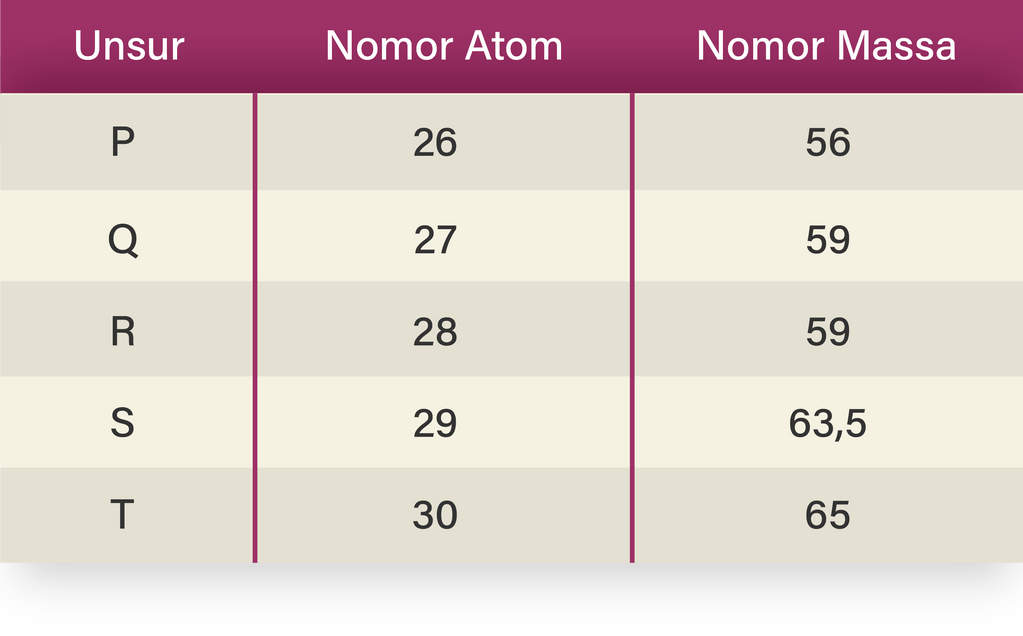
Diberikan tabel nomor atom dan nomor massa unsur...
Massa atom (m a) dari suatu unsur kimia adalah massa suatu atom pada keadaan diam, umumnya dinyatakan dalam satuan massa atom. [1] Massa atom sering disinonimkan dengan massa atom relatif, massa atom rata-rata, dan bobot atom. Walaupun demikian, terdapat sedikit perbedaan karena nilai-nilai tersebut dapat berupa rata-rata berbobot dari massa.

3 Modi per Calcolare la Massa Atomica wikiHow
The atomic mass unit (abbreviated u, altho ugh amu is a lso used) is defined as 1/12 of the mass of a 12C atom: 1 u = 1 12 the mass of 12Catom (2.6.1) (2.6.1) 1 u = 1 12 the mass of 12 C a t o m. It is equal to 1.661 × 10 −24 g. Masses of other atoms are expressed with respect to the atomic mass unit.

Massa Atom Relatif, Berikut Penjelasan, Rumus, dan Cara Menghitungnya
Fosfor. Dari bahasa Yunani phosphoros, pembawa cahaya; nama lama untuk planet Venus yang muncul sebelum matahari terbit. Hennig Brand discovered phosphorus in 1669, in Hamburg, Germany, preparing it from urine. In 1769, Johan Gottlieb Gahn and Carl Wilhelm Scheele showed that calcium phosphate is found in bones, and they obtained elemental.

Contoh Nomor Atom Dan Nomor Massa
Atomic mass of Phosphorus is 30.9738 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom.