
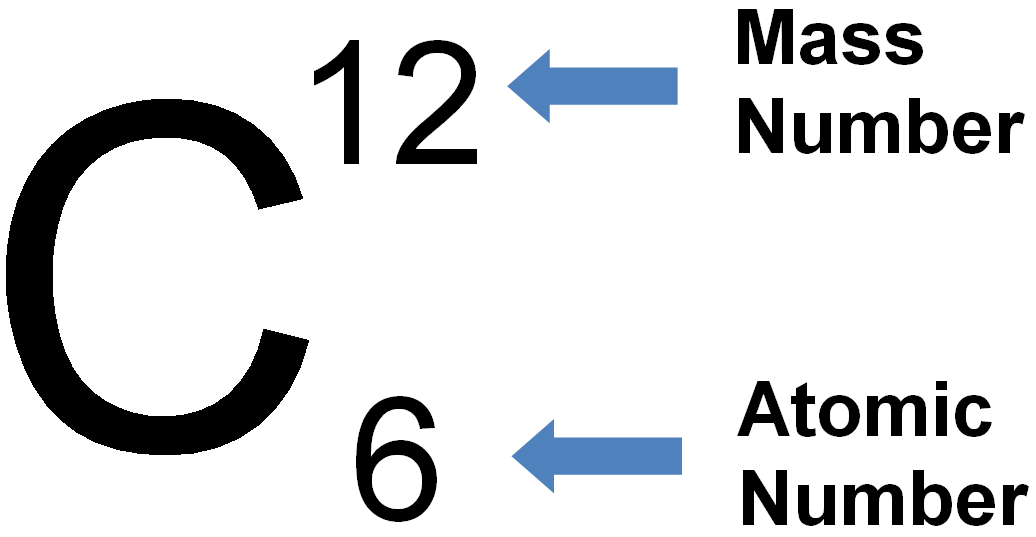
Perbedaan Nomor Atom Dan Nomor Massa Rumus Kimia IMAGESEE
Hukum Dasar Kimia Kelas 10 - Hukum dari Para Ahli & Contoh Soalnya. by sereliciouz & Andjar Tyassih, S.Si. Agustus 13, 2019. Hukum dasar Kimia dipelajari untuk mengerti cara kerja dan konsep dalam dunia kimia. Ada beberapa hal yang harus dipelajari sebelum mengerti hukum dasar Kimia, yakni massa atom relatif dan massa molekul relatif.
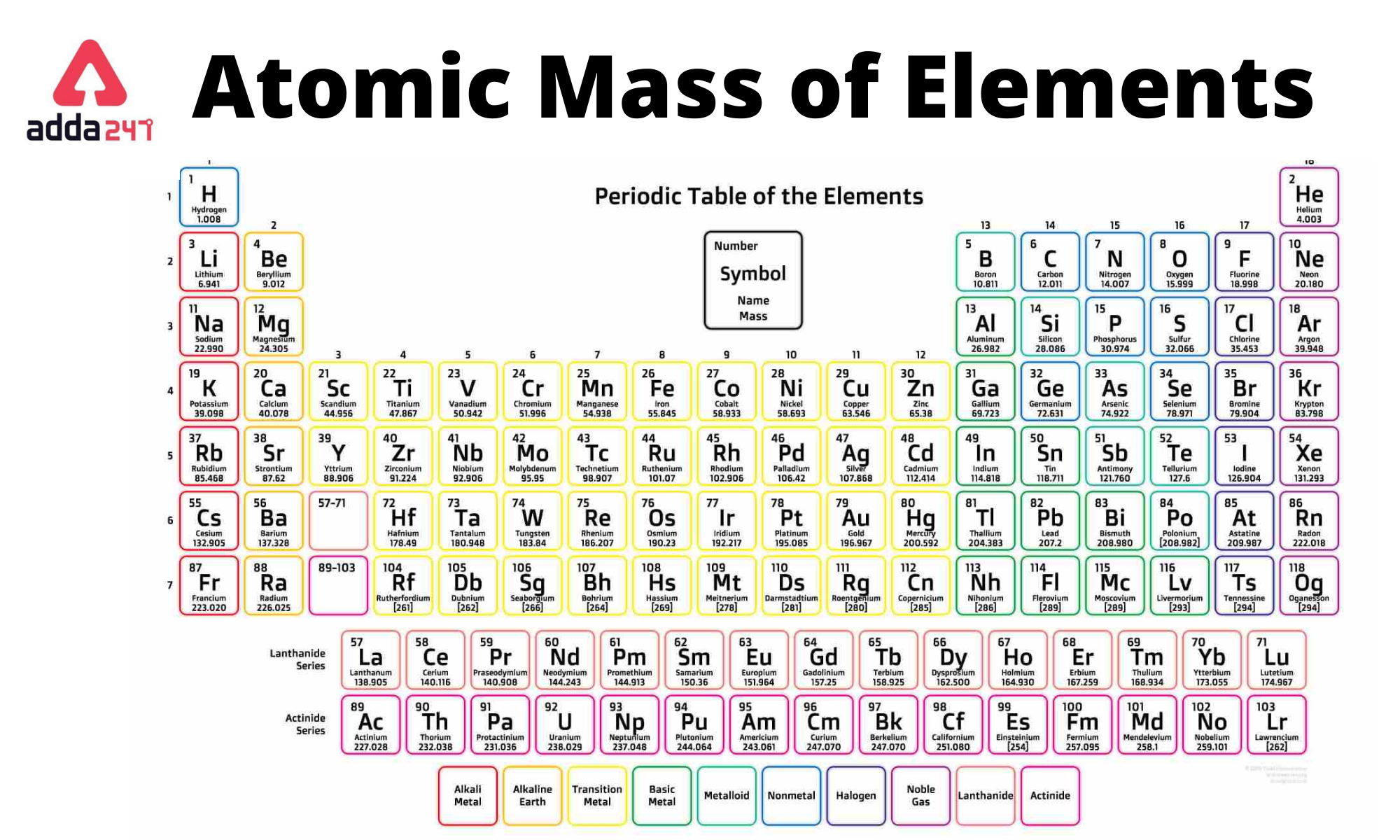
Periodic Table Element With Atomic Mass And Atomic Number
Introduction. In chemistry, there are many different concepts of mass. It is often assumed that atomic mass is the mass of an atom indicated in unified atomic mass units (u).However, the book Quantities, Units and Symbols in Physical Chemistry published by the IUPAC clearly states: "Neither the name of the physical quantity, nor the symbol used to denote it, should imply a particular choice of.

Massa Atom Relatif, Berikut Penjelasan, Rumus, dan Cara Menghitungnya
Atomic mass of Carbon is 12.0107 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or.
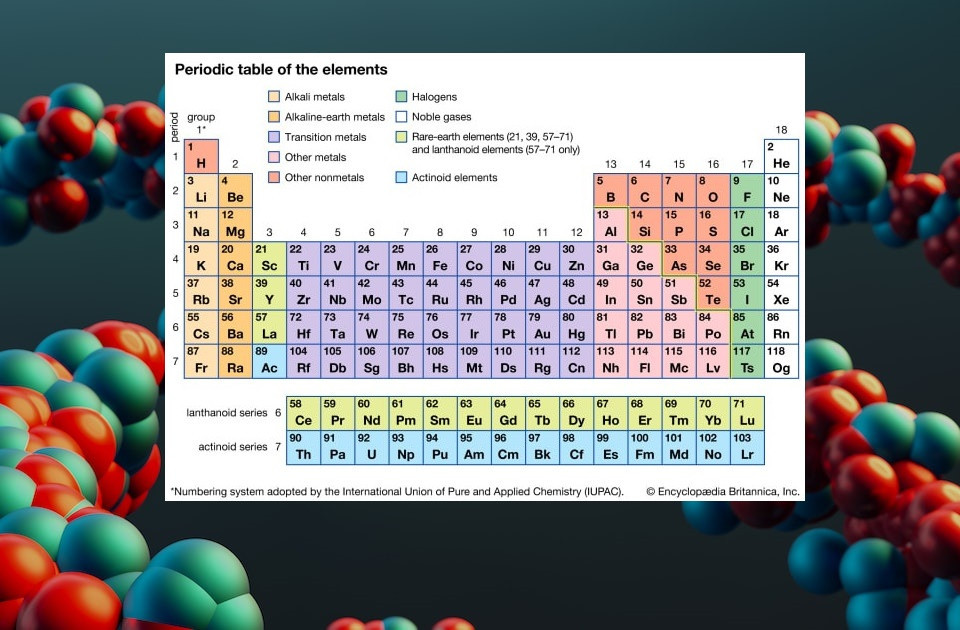
Modern Periodic Table With Atomic Mass And Atomic Number
Contoh Soal Massa Atom Relatif dan Pembahasannya. Belajar rumus massa atom relatif kurang lengkap kalau tidak disertai contoh soal dan pembahasannya. Yuk, simak beberapa contoh soal massa atom relatif berikut ini supaya kamu semakin paham. Contoh 1. Jika Ar Fe = 56 sma dan massa 1 atom 12 C = 2 x 10-23 gram, tentukan massa 5 atom besi. Pembahasan
Cara Mencari Nomor Atom dan Nomor Massa, Lengkap dengan Contohnya
Video ini berisi cara menentukan massa atom relatif ( Ar) berdasar standar masa atom C 12, dan berdasar isotop & kelimpahan di alam. -----.

Tabel Massa Atom Relatif
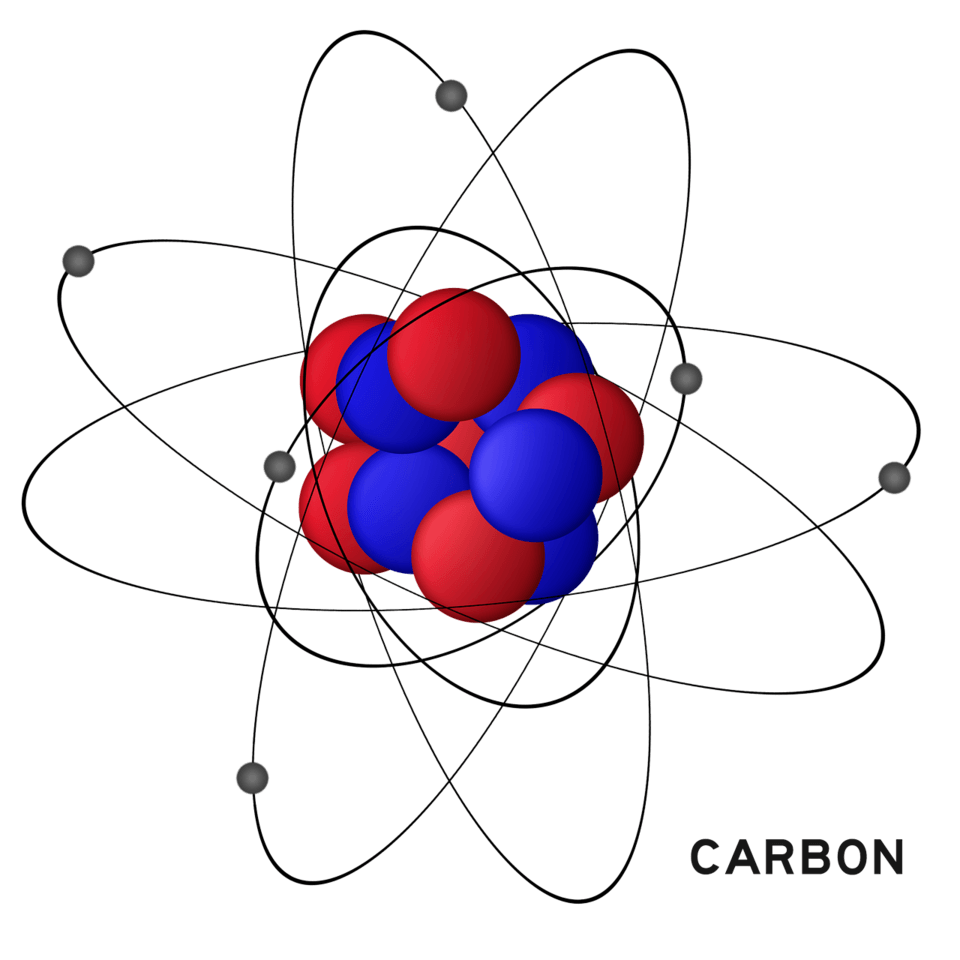
Karbon. Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. In 1772, Antoine Lavoisier showed that diamonds are a form of carbon; when he burned samples of charcoal and diamond and found that neither produced any water. In 1779, Carl Wilhelm Scheele showed that graphite burned.

3 Modi per Calcolare la Massa Atomica wikiHow
The atomic mass unit (u or amu) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amu's (u's). This is the standard unit for atomic or molecular mass, and 1 amu is thus 1/12 th the mass of a 12 C atom. This is obviously very small. 1 amu = 1.66054x10-27Kg = 1.66054x10-24 g.

Massa Atom Relatif dan Jarak Antar Atom berdasarkan Teori Fisika Materi Ahmad Dahlan
An atomic mass unit is defined as a mass equal to one twelfth the mass of an atom of carbon-12. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. For example, one atom of helium-4 has a mass of 4.0026amu 4.0026 amu. An atom of sulfur-32 has a mass of 31.972 amu 31.972 amu.

Lengkapi tabel berikut. Nomor massa Nomor atom Jumlah Pro...
Dalton atau satuan massa atom terpadu (simbol: Da atau u) adalah satuan massa yang banyak digunakan dalam fisika dan kimia. Ini didefinisikan sebagai 1/12 massa atom karbon-12 netral tak terikat dalam keadaan dasar nuklir dan elektroniknya dan saat diam. Konstanta massa atom, dilambangkan m u, didefinisikan secara identik, memberi m u = m(12 C)/12 = 1 Da.. Satuan ini biasanya digunakan dalam.
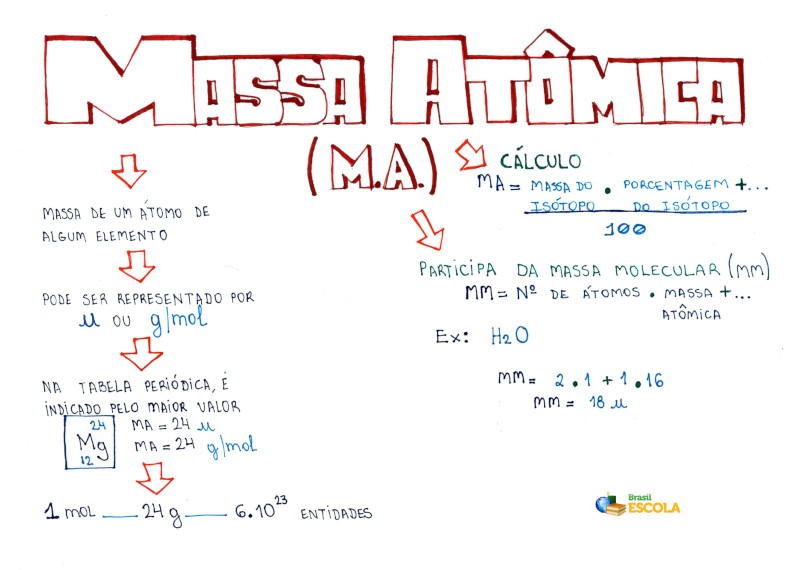
O que é massa atômica? Brasil Escola
Relative atomic mass (symbol: A r; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant.The atomic mass constant (symbol: m u) is defined as being 1 / 12 of the mass of a carbon-12 atom.

3 Modi per Calcolare la Massa Atomica wikiHow
There are two steps to find the mass of the Carbon (C) atom. First we find the atomic mass of C from the Periodic Table. We then divide this by Avogadro's N.
Massa atomica immagini e fotografie stock ad alta risoluzione Alamy
Karena dalam soal sudah memakai s.m.a, Jadi kalian tinggal masukin aja deh massa atom C-12 dengan satuan s.m.a. Nah, akhirnya dapet deh jawabannya sebesar 35 s.m.a, tapi biasanya dalam soal kalian akan disuguhkan dalam satuan metrik seperti kilogram atau gram, sehingga kalian harus menghitungnya dengan teliti dan cermat!

Diketahui massa atom C12 lebih ringan dibandingkan denga...
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
Resumo Sobre Massa atômica e massa molecular Química Projeto Sophia
Karbon (dari bahasa Latin: carbo "arang") atau zat arang adalah sebuah unsur kimia dengan lambang C dan nomor atom 6. Ia merupakan nonlogam dan tetravalen — atomnya membuat empat elektron tersedia untuk membentuk ikatan kimia kovalen. Ia berada dalam golongan 14 dari tabel periodik. [12] Karbon hanya menyusun sekitar 0,025 persen dari kerak.

Tabel Massa Atom Relatif Dan Bilanganoksidasi Unsur
Konsep Mol Kimia Kelas 10 - Pengertian, Konsep, dan Latihan Soal. by sereliciouz & Andjar Tyassih, S.Si. Agustus 13, 2019. Konsep mol kimia dalam Quipper Blog kali ini akan dibahas konsep persamaan reaksi, penyetaraan persamaan reaksi, persentase massa unsur, pengertian mol, massa molar, volume molar, dan contoh soal.

MASSA ATOM RELATIF (Ar) DAN MASSA MOLEKUL RELATIF YouTube
Atomic mass is expressed as a multiple of one-twelfth the mass of the carbon -12 atom, 1.992646547 × 10 −23 gram, which is assigned an atomic mass of 12 units. In this scale, 1 atomic mass unit (amu) corresponds to 1.660539040 × 10 −24 gram. The atomic mass unit is also called the dalton (Da), after English chemist John Dalton. The.