
33.THE ATOMIC STRUCTURE Schrödinger Equation. madoverchemistry
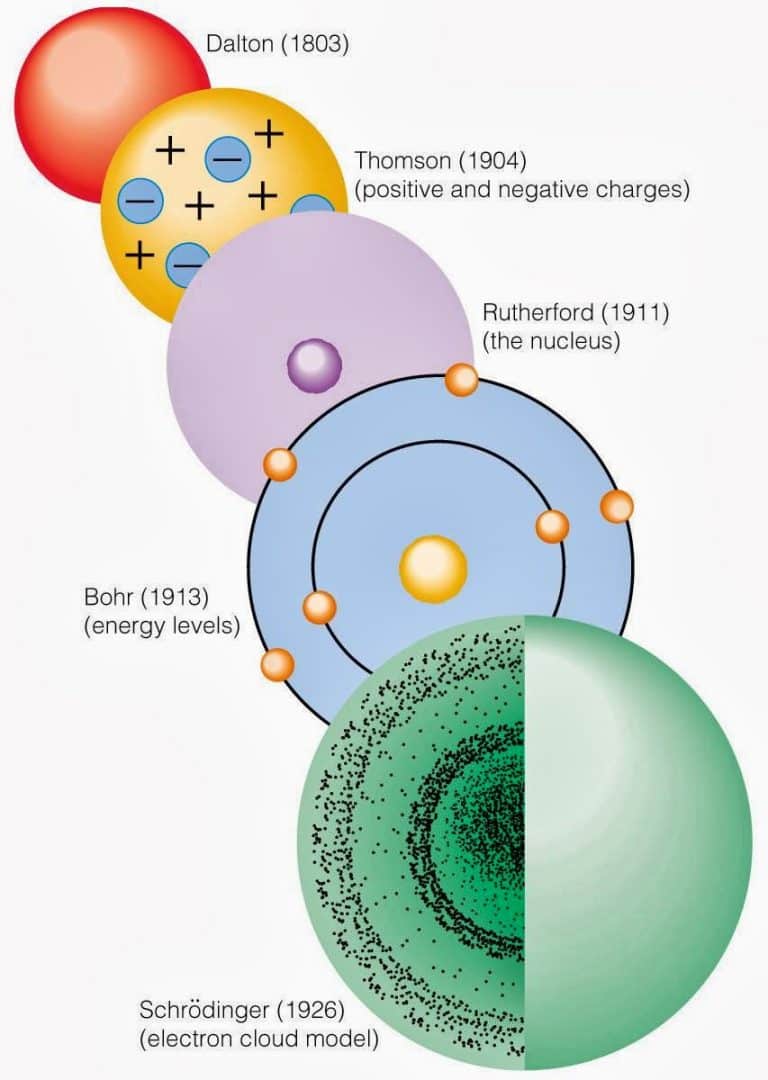
The theory provided a good description of the spectrum created by the hydrogen atom, but needed to be developed to suit more complicated atoms and molecules. Assuming that matter (e.g., electrons) could be regarded as both particles and waves, in 1926 Erwin Schrödinger formulated a wave equation that accurately calculated the energy levels of electrons in atoms.
Bohr and Schrodinger's Atomic Models Science, Chemistry, Atoms ShowMe
Teori Mekanika Kuantum yang dikembangkan Erwin Schrodinger, Max Planck & ahli lainnya, hingga model atom mekanika kuantum dibahas lengkap di artikel ini. Yuk, belajar bareng!. Model atom mekanika kuantum (sumber gambar: www.sutori.com) Sebelum lanjut, yuk download dulu aplikasi Zenius. Elo bisa dapetin akses ke ribuan materi pelajaran.
Modelo atômico de Schrödinger Definição e principais características
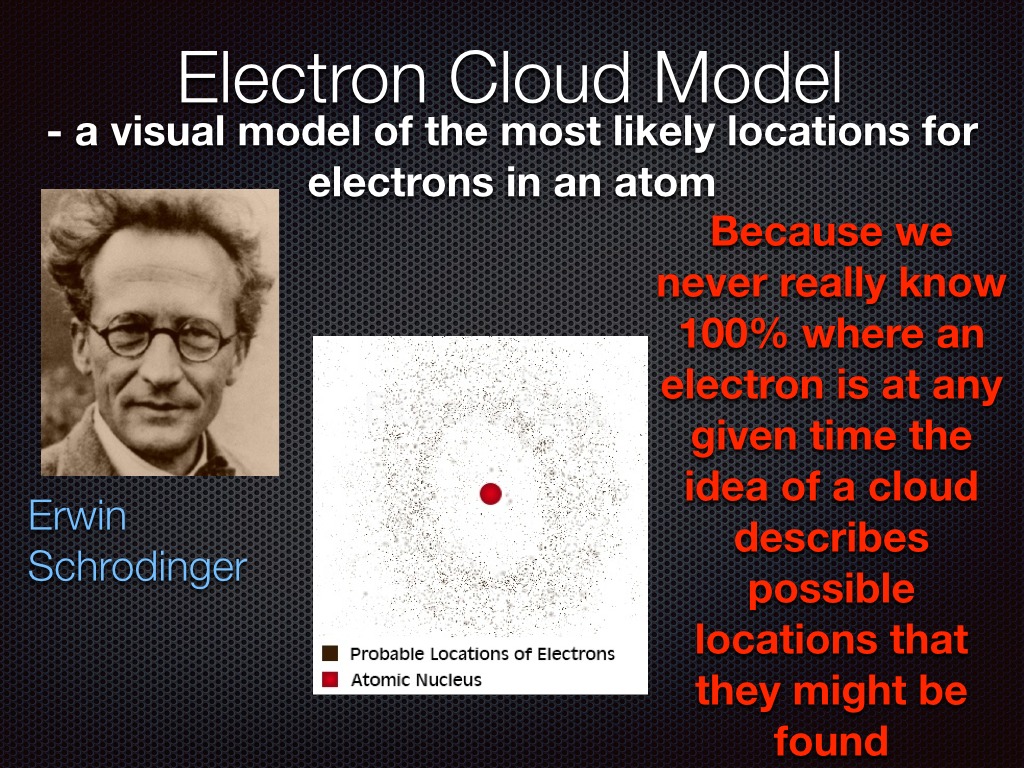
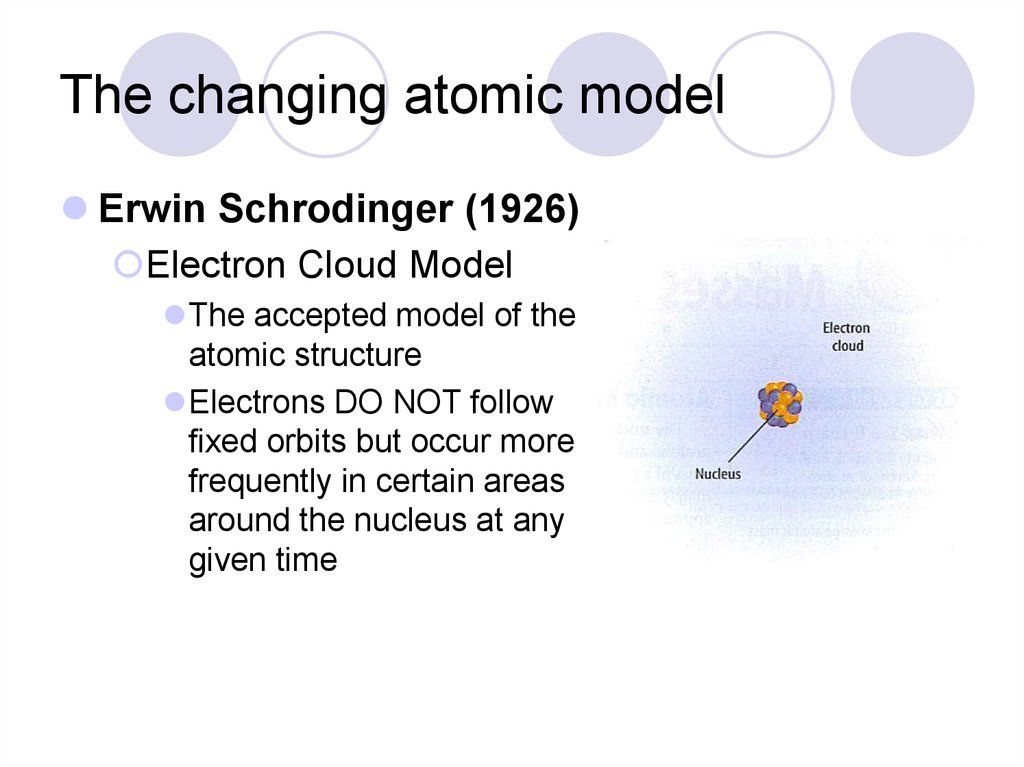
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position. This atomic model is known as the quantum mechanical model of the atom. Unlike the Bohr model, the quantum mechanical model does not.

Kelebihan Dan Kelemahan Teori Atom Modern bintangutama69.github.io
Figure 3.1. 1: Schrödinger's cat: a cat, a flask of poison, and a radioactive source are placed in a sealed box. If an internal monitor detects radioactivity (i.e., a single atom decaying), the flask is shattered, releasing the poison, which kills the cat. The Copenhagen interpretation of quantum mechanics implies that after a while, the cat.
Perhatikan Gambar Model Atom Berikut
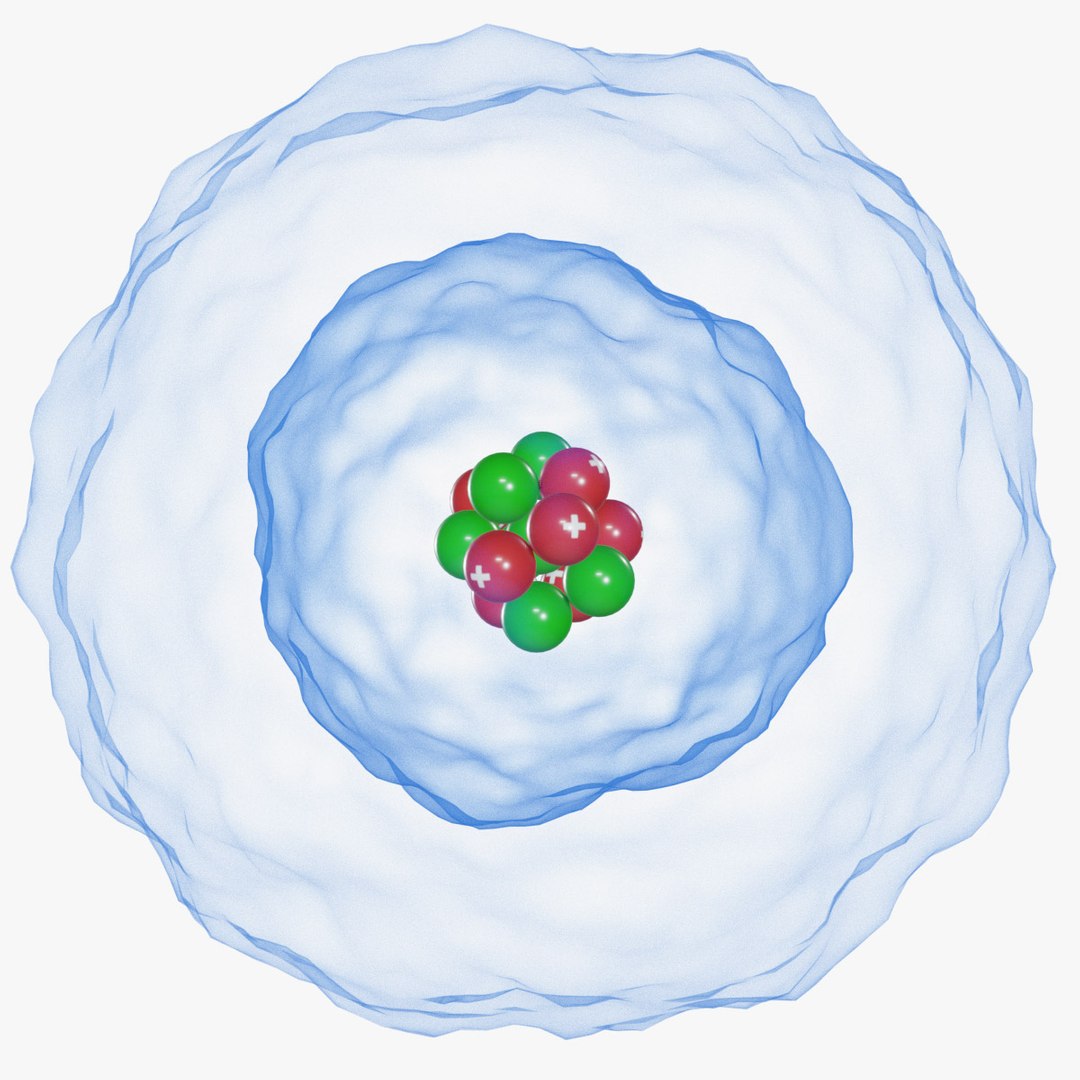
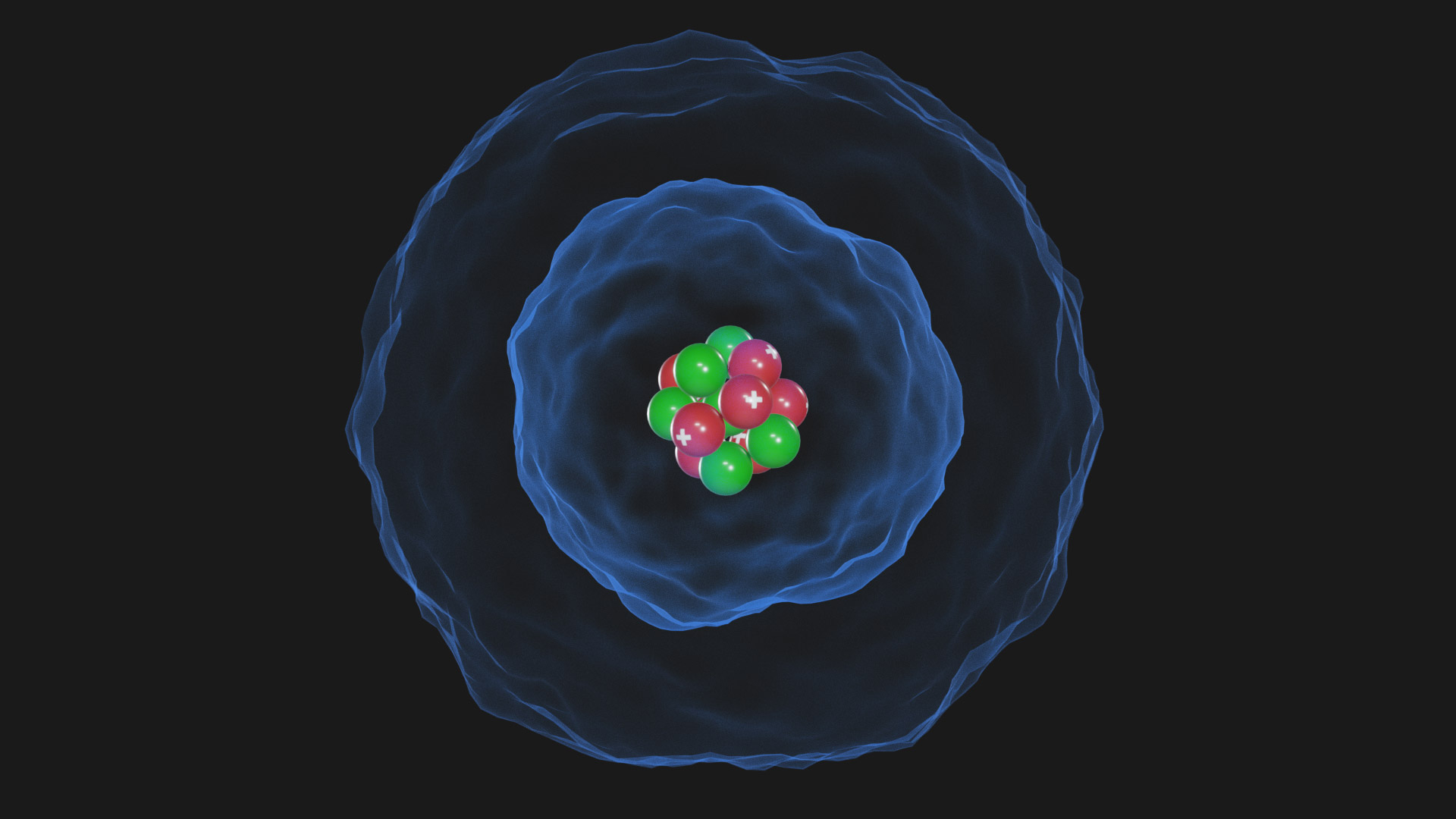
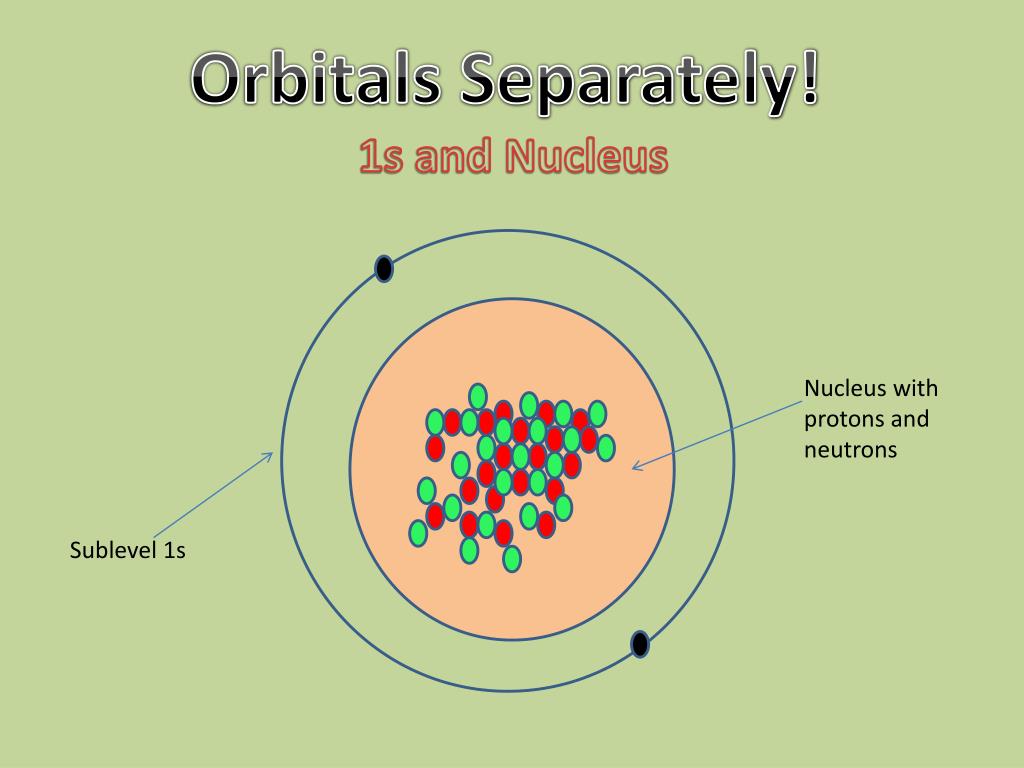
The Erwin Schrödinger model of the atom is composed of the nucleus of the atom which contains protons and neutrons and is surrounded by an electron cloud. This is sometimes called the cloud model.
PPT The Atomic Model Through Time PowerPoint Presentation, free download ID5318415
Answer link. The model is known as the electron cloud model or the quantum mechanical model of an atom. The wave equation that he proposed upon being solved gives us a set of three integral numbers known as quantum numbers to specify the wave function of an electron. It was revealed that later a fourth quantum number i.e. the spin quantum.
Schrodinger atomic model genuinenanax
5: The Quantum Model of the Atom. Page ID. 465523. In the early 1930's Erwin Schrödinger published a way of thinking about the circumstance of radioactive decay that is still useful. We imagine an apparatus containing just one Nitrogen-13 atom and a detector that will respond when the atom decays. Connected to the detector is a relay connected.
6. Erwin Schrodinger (18871961) Atomic Theory Timeline
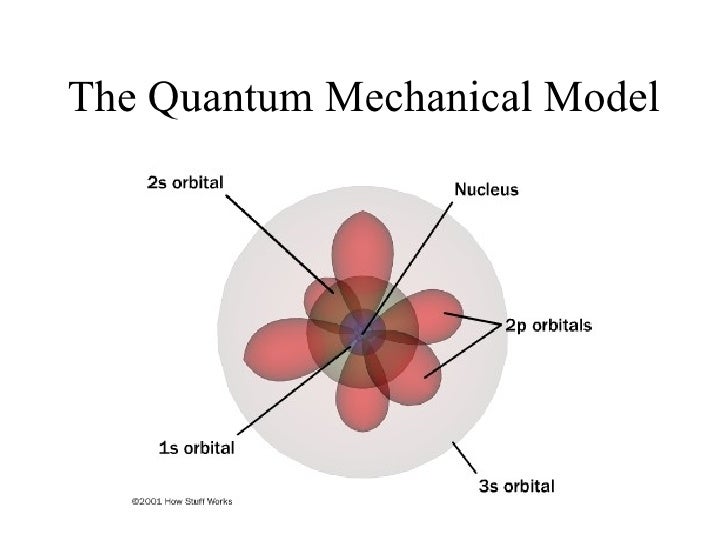
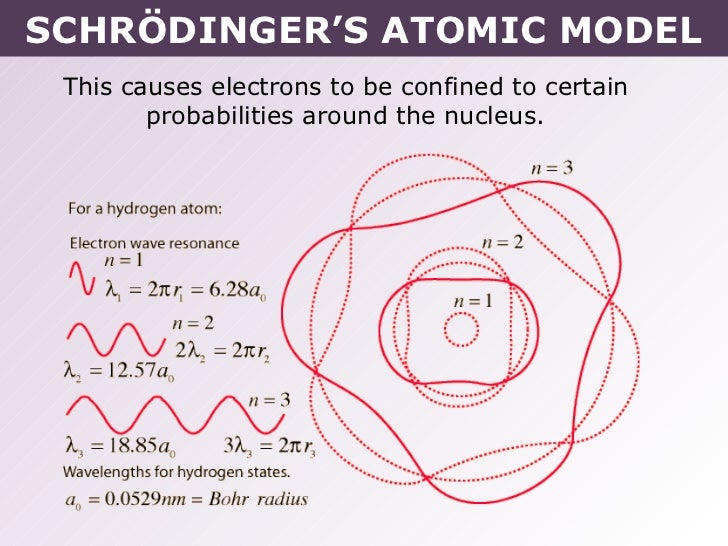
given by the following equation: λ = h m v. Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, H ^ ψ = E ψ. . , can be solved to yield a series of wave function ψ. . , each of which is associated with an electron binding energy, E. .

A Century of Quantum Physics Erwin Schrodinger
A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie equation to generate a mathematical model for the distribution of electrons in an atom. The advantage of this model is that it consists of mathematical equations known as wave functions that.

Schrödinger's Contribution to the Model of the Atom HSC Physics Science Ready
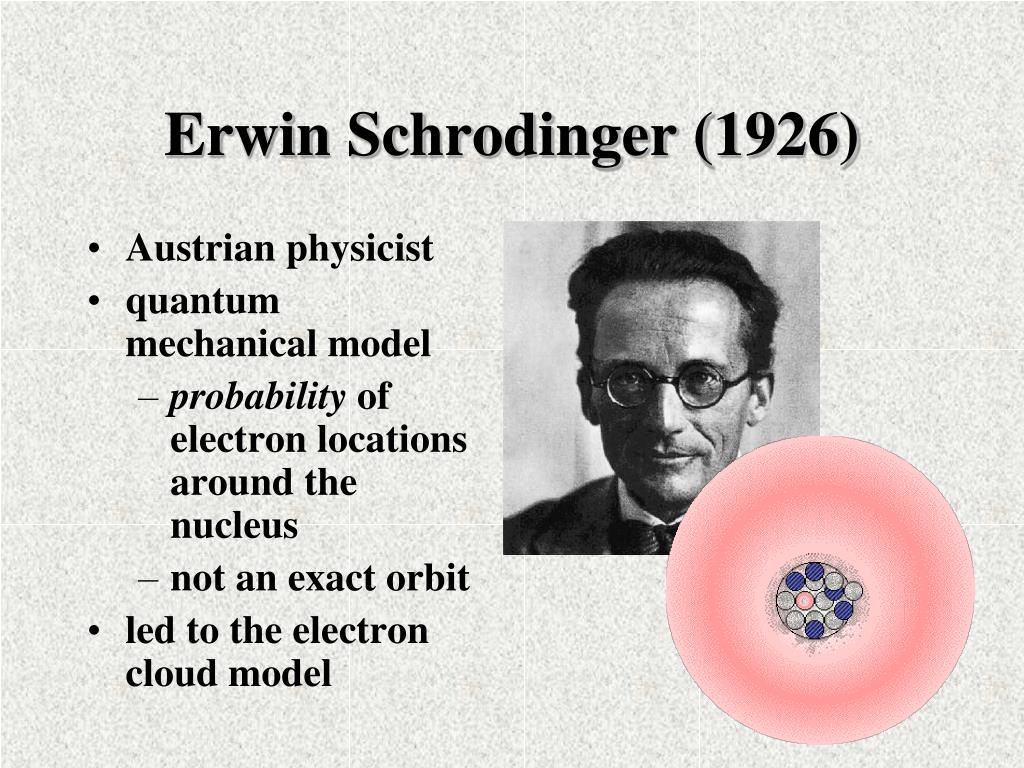
Erwin Schrödinger (born August 12, 1887, Vienna, Austria—died January 4, 1961, Vienna) Austrian theoretical physicist who contributed to the wave theory of matter and to other fundamentals of quantum mechanics. He shared the 1933 Nobel Prize for Physics with British physicist P.A.M. Dirac.
Tang 02 schrödinger’s atomic model
The model of the electron in the nucleus was further developed by the work of Erwin Schrodinger and his development of his wave equation. This equation could be used to solve and give an accurate description of the energy of an electron in an atom due to vibrational modes and the creation of peaks and troughs when these electrons are treated as waves.
3D Atom Quantum Model Schrodinger model TurboSquid 1770104
📖 Visit our website: http://www.scienceready.com.au 🏅 Become a Patron: https://www.patreon.com/scienceready🎶 Follow our Tiktok https://www.tiktok.com/@hs.
3D Atom Quantum Model Schrodinger model TurboSquid 1770104
Quantum Mechanical Atomic Model. In 1926, Austrian physicist Erwin Schrödinger (1887-1961) used the wave-particle duality of the electron to develop and solve a complex mathematical equation that accurately described the behavior of the electron in a hydrogen atom. The quantum mechanical model of the atom comes from the solution to.
Schrodinger's Model
Erwin was born August 12, 1887 in Austria. He attended the University of Vienna and after attending this college in 1926 her came up with his own wave equation to explain the distribution of electrons in an atom.. In 1926, Schrödinger took the Bohr Atom model further and calculated a mathematical equation to discover the probability of an.
PPT Erwin Schrödinger’s Model of the Atom Element Representation PowerPoint Presentation ID
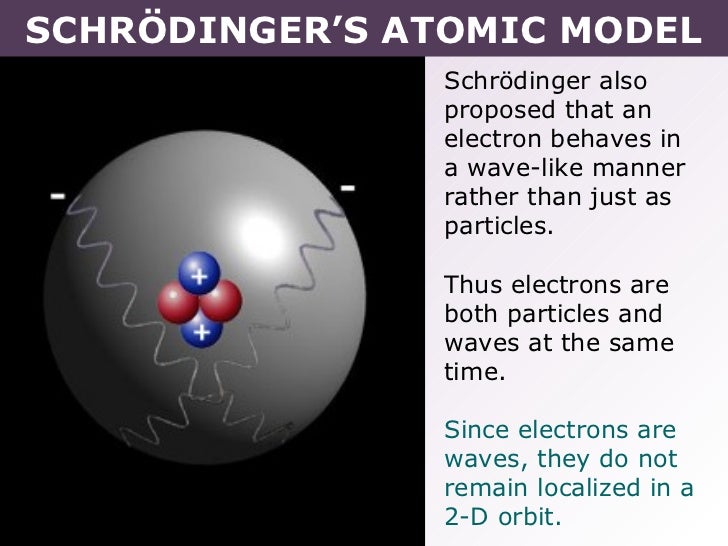
Schrödinger's Contribution to the Current Model of the Atom. Schrödinger used de Broglie's matter wave theory to develop a probabilistic model of the atom. In Schrödinger's model, electrons do not follow sharply defined orbits (like in Bohr's model), but rather are found in orbitals. In addition, Schrödinger's atomic model is based on.

Modelo Atómico de Schrödinger Teoría Atomica
QUICK FACTS. Name: Erwin Schrödinger. Birth Year: 1887. Birth date: August 12, 1887. Birth City: Vienna. Birth Country: Austria. Gender: Male. Best Known For: Erwin Schrödinger was a Nobel Prize.