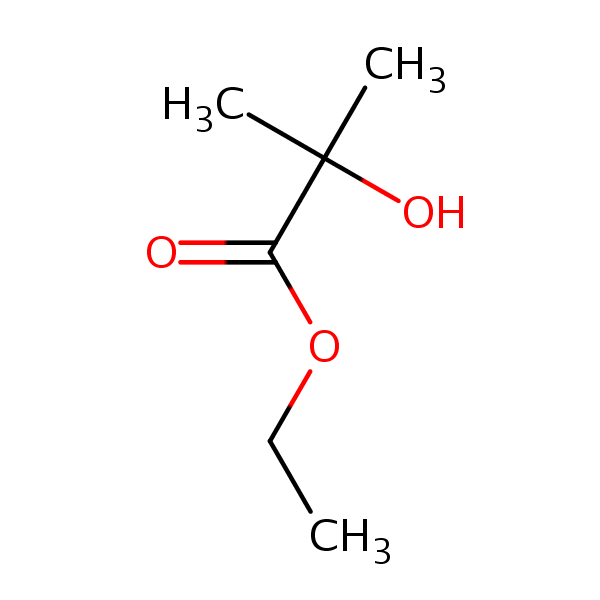
Propanoic acid, 2hydroxy2methyl, ethyl ester SIELC Technologies
A new [4+2] cycloaddition-based strategy from thiophene ring was reported. The reaction of annulated thiophenes 221a and 221b with DMAD 51 and with ethyl propionate 222 in refluxing dioxane afforded thermally stable condensed thiepines 226 in 74-80% yield ( Scheme 30) <1996T11915>. The reaction of 227a and 227b with DMAD 51 in refluxing.
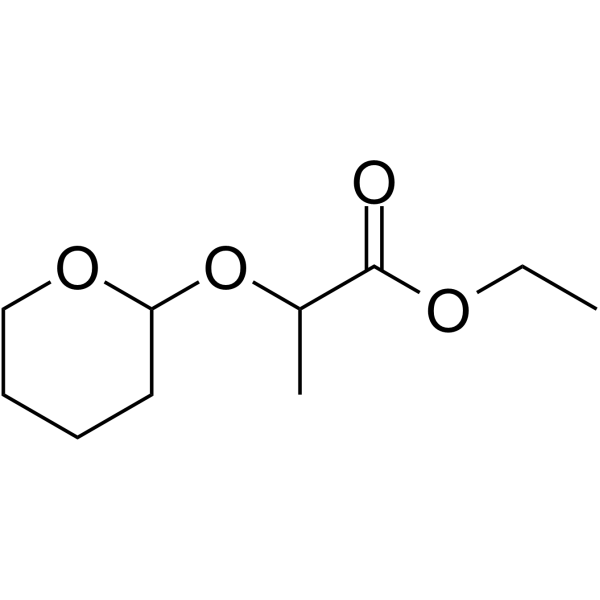
THPCH3ethyl propionate PROTAC Linkers MedChemExpress
Ethyl propionate is a common volatile found in fresh citrus fruits and juices, some whiskeys, and in Parna ham. If released to air, a vapor pressure of 35.8 mm Hg at 25 °C indicates ethyl propionate will exist solely as a vapor in the ambient atmosphere.
PPT Organic Reactions PowerPoint Presentation, free download ID3839223
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named by changing the ending of the parent alkane name to - ol. Alcohols can be considered derivatives of water (H 2 O; also written as HOH). Like the H-O-H bond in water, the R-O-H bond is bent, and alcohol molecules are polar.
[Solved] Ethyl propanoate structural formula Course Hero
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others. Contact Technical Service. Aldrich-112305; Ethyl propionate 0.99; CAS Number: 105-37-3; Linear Formula: CH3CH2COOC2H5; find related products, papers, technical documents, MSDS.

1. Ethanoic anhydride to ethanol. 2. Ethyl propanoate to ethanol. 3 ethyl propanoate to propanol
IUPAC Standard InChIKey: FKRCODPIKNYEAC-UHFFFAOYSA-N Copy CAS Registry Number: 105-37-3 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: Propionic acid, ethyl ester; Ethyl propanoate; Ethyl propionate; Propionic ester; Propionic ether; C2H5COOC2H5; Propionate d'ethyle.

Ethyl propionate d5 (Ethyl propanoate d5) EPTES
53630283. Structure. Molecular Formula. C5H8O2. Molecular Weight. 100.12 g/mol. Computed by PubChem 2.1 (PubChem release 2021.05.07) Parent Compound.

Ethyl 2Methyl2(methyltellanyl)propanoate 474094069 梯希爱(上海)化成工业发展有限公司
Other names: Propionic acid, ethyl ester; Ethyl propanoate; Ethyl propionate; Propionic ester; Propionic ether; C2H5COOC2H5; Propionate d'ethyle; Ethylester kyseliny propionove; UN 1195; Ethyl ester of propanoic acid; Ethyl n-propionate; n-Ethyl propanoate; NSC 8848 Permanent link for this species. Use this link for bookmarking this species for.
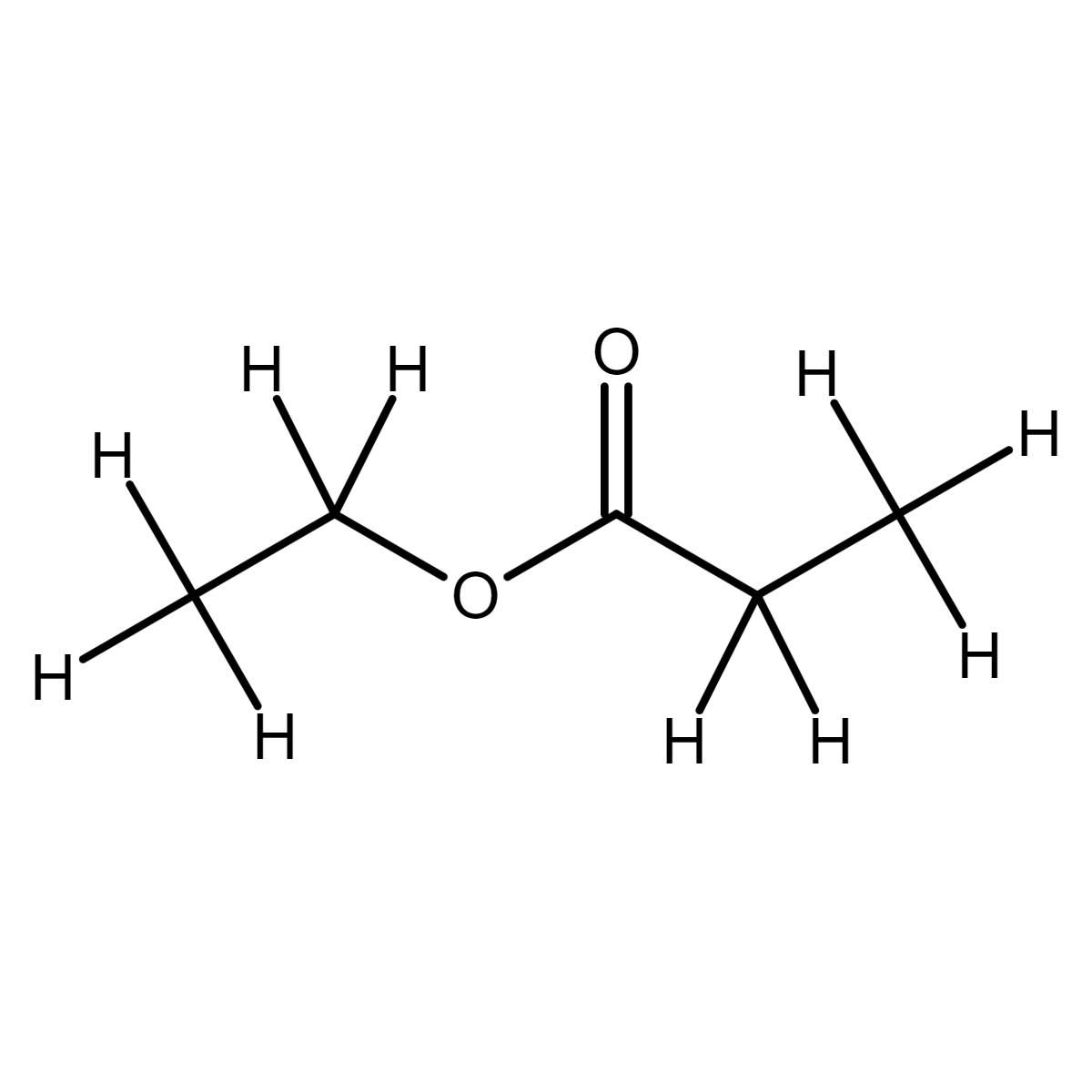
Ethyl Propionate CRM LABSTANDARD
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The ETHYL PROPIONATE molecule contains a total of 16 bond (s). There are 6 non-H bond (s), 1 multiple bond (s), 3 rotatable bond (s), 1 double bond (s), and 1 ester (s) (aliphatic). Images of the chemical structure of ETHYL.

Liquid Ethyl Propionate EP / CAS No.105373, Grade Standard Technical Grade, Rs 165
Propanoic acid, ethyl ester. Formula: C 5 H 10 O 2. Molecular weight: 102.1317. IUPAC Standard InChI: InChI=1S/C5H10O2/c1-3-5 (6)7-4-2/h3-4H2,1-2H3. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: FKRCODPIKNYEAC-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet.
What is the industrial preparation of esters (ethyl ethanoate)? Quora
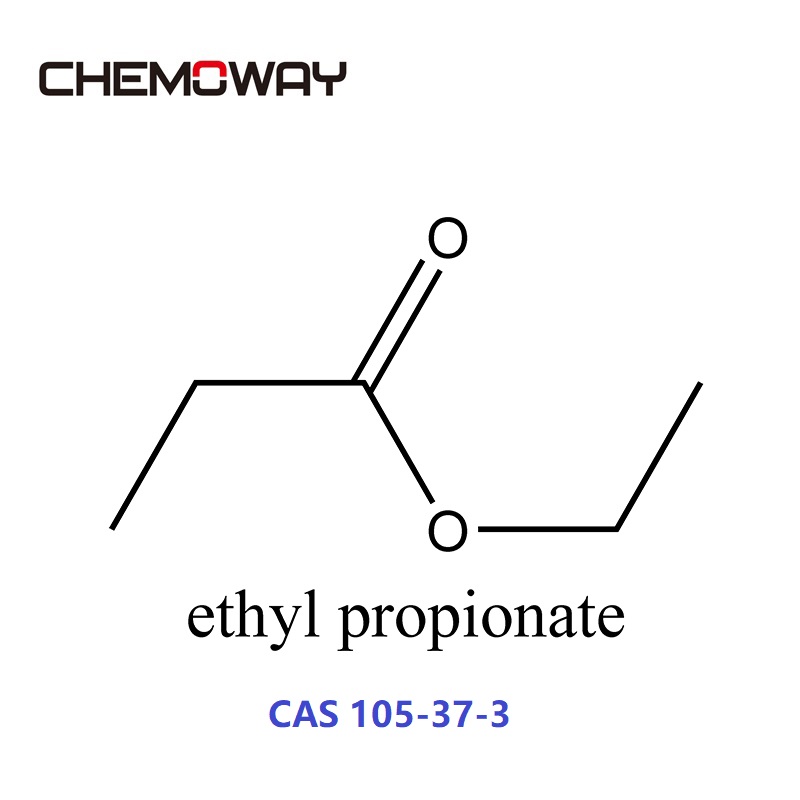
Ethyl propionate. Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Ethyl propionate is an organic compound with formula C 2 H 5 O 2 CCH 2 CH 3. It is the ethyl ester of propionic acid. It is a colorless volatile liquid with a pineapple-like odor. [3]

Which of the following compound is formed as major product when ethyl propanoate and ethyl
Figure 5.6. 1 shows models for two common esters. Figure 5.6. 1: The Structure of Esters. Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom, which is then joined to an alkyl or an aryl group. The esters shown here are ethyl acetate (a) and methyl butyrate (b).
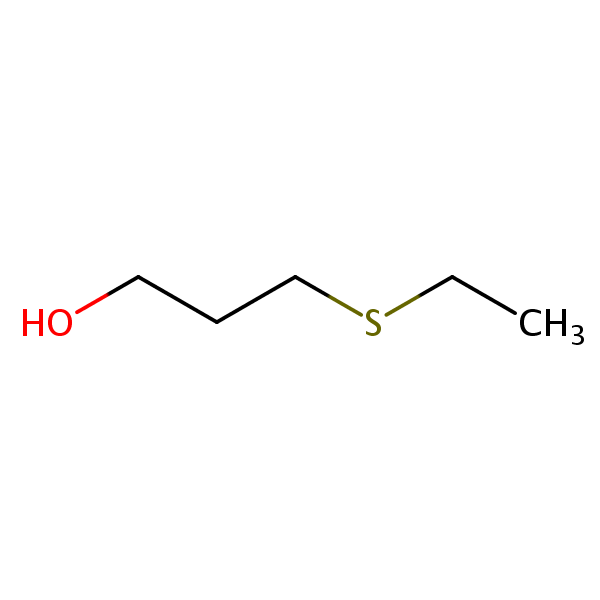
3(Ethylthio)propanol SIELC
Organic Chemistry IntroductionDrawing Esters Step-by-Step Tutorial!Esters have two components. One component is from the carboxylic acid (parent chain, which.

Rumus Kimia Etil Propanoat Bit CDN
ethyl propanoate: CH 3 CH 2 COOCH 2 CH 3: 1.7: The reason for this trend in solubility is that although esters cannot hydrogen bond with each other, they can hydrogen bond with water molecules. One of the partially-positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on one of the oxygen atoms in an.

Fuel destruction pathways for ethyl propanoate. Published on 15 March... Download Scientific
An alkyl group (in green) is attached directly to the oxygen atom by its middle carbon atom; it is an isopropyl group. The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. The ester is therefore isopropyl benzoate (both the common name and the IUPAC name). Exercise 9.8.1 9.8. 1.
ethyl
NIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 - Professional Edition Version 2-2012-1-Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics. These data were generated through dynamic data analysis, as implemented in the NIST ThermoData Engine software.

1Iodo2methylpropane, 97, stabilized, Thermo Scientific Chemicals, Quantity 25 mL Fisher
In Greek-style samples there was a meaningful increase on time of ethyl-propanoate, 2-butanone and propionic acid contents (Figure 24.7) but, on the other hand, many molecules increased their contents at 7 months and diminished at 8 months (Table 24.1).