
Formula Molecular Y Estructural Del Eter Etil Etilico Formă Blog
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula C 4 H 10 O, (CH 3 CH 2) 2 O or (C 2 H 5) 2 O, sometimes abbreviated as Et 2 O. It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid.It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general.

Metil tertbutil éter (MTBE, tbme) el aditivo de gasolina molécula fórmula esquelética Imagen
Metoxietano. (25 ℃ y 1 atm ), salvo que se indique lo contrario. El metoxietano, también denominado etil metil éter, es un compuesto orgánico perteneciente al grupo de los éteres y formado por un grupo etilo unido a un radical metoxilo. Se trata de un gas incoloro con olor a medicina; se caracteriza por ser altamante inflamable y por.
Metiltbutil etere Wikipedia
Preparation of Esters. The most versatile method for the preparations of esters is the nucleophilic acyl substitution of of an acid chloride with an alcohol. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters.
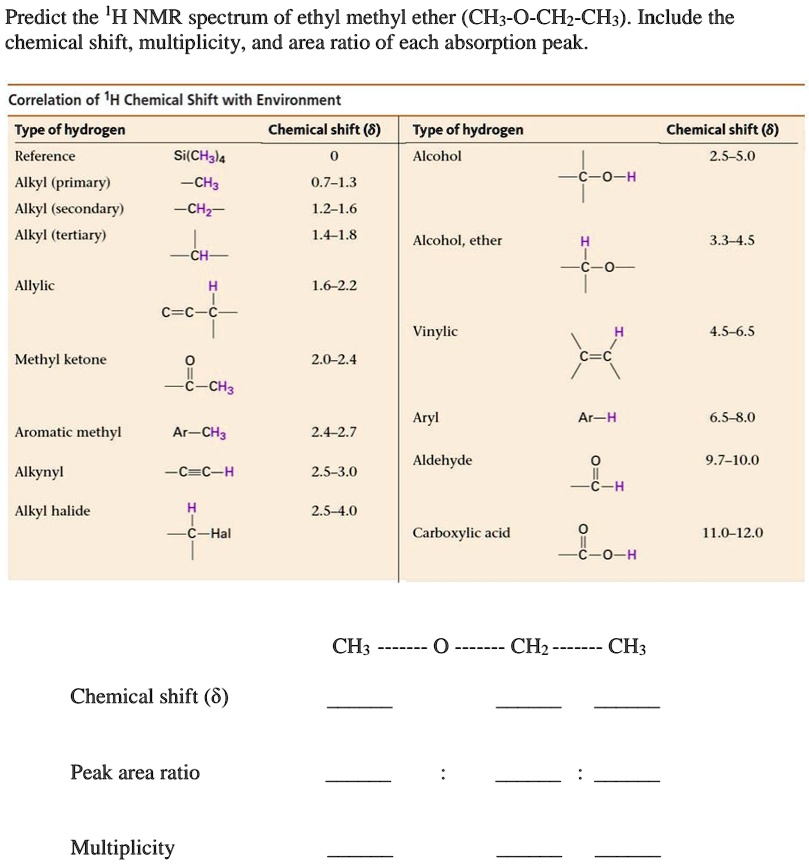
SOLVED Predict the 'H NMR spectrum of ethyl methyl ether (CHz0CHzCH3). Include the chemical
Under basic conditions, the mechanism begins with the nucleophilic reaction of the alkoxide with the carbonyl carbon to produce the tetrahedral intermediate. The carbonyl reforms with the loss of the leaving group to produce a new ester. 1) Nucleophilic reaction by an alkoxide. 2) Leaving group removal.

エチルメチルエーテル ethyl methyl ether
Productos adecuados para etil metil éter C 3 H 8 O. Tanto si necesita detectores de gases portátiles, como tubos de detección de gases o equipos de protección individual, Dräger cuenta con un completo catálogo de productos para protegerle a la hora de manipular sustancias peligrosas. Máscaras & Filtros Póngase en contacto con nosotros.
What is the action of hot and cold HI on ethyl methyl ether? Give
Allyl ethyl ether 95%; CAS Number: 557-31-3; EC Number: 209-169-7; Synonyms: 3-Ethoxypropene,Ethyl allyl ether; Linear Formula: C2H5OCH2CH=CH2; find Sigma-Aldrich-238228 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
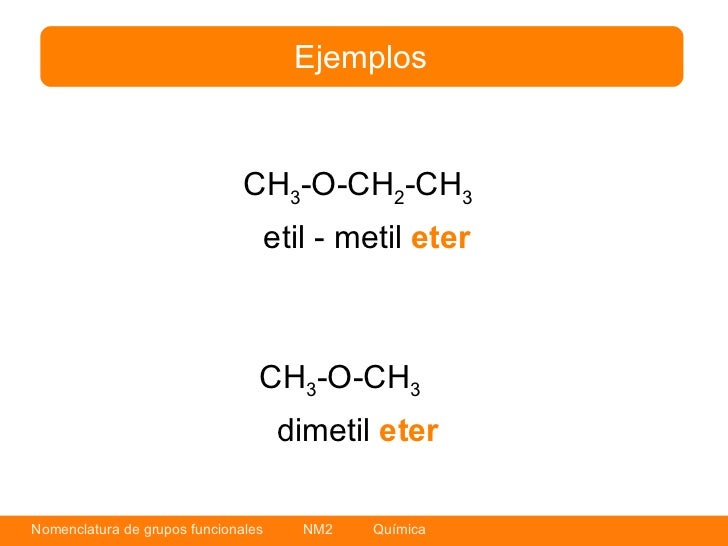
Nomenclatura grupos funcionales quimica organica
Ez azt bizonyítja, hogy a kénsav és az etil-alkohol a hőmérséklettől függően különböző módon reagál egymással. A vegyes éterekhez egy alkohol nátrium sójára is szükség van. Például etil-alkohol nátriummal való reakciójából hidrogénfejlődés mellett keletkezik nátrium-etoxid, majd ezt például metil-alkohollal.
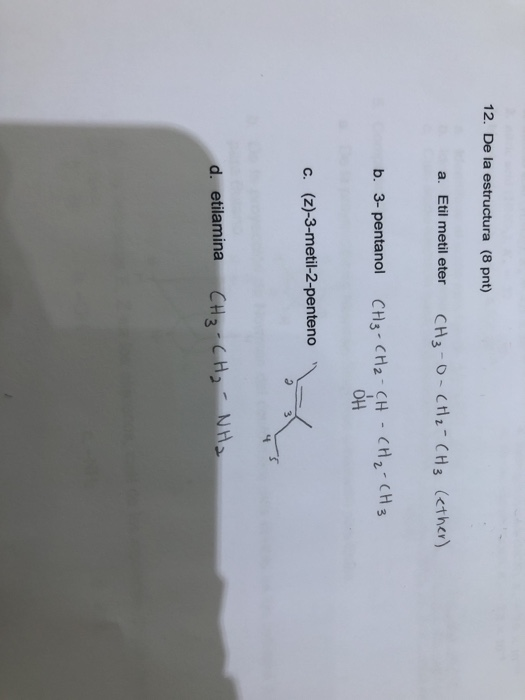
Solved 12. De la estructura (8 pnt) a. Etil metil eter
Előállítása. A dimetil-étert legnagyobb mennyiségben metanolból állítják elő vízelvonással, kondenzációs reakcióval. Ekkor két molekula metanolból egy molekula víz kilépésével dimetil-éter keletkezik. A vízelvonást savak katalizálják. Előállítható az éterek többi általános előállítási módszerével is.
Nomenclatura del eter
Las principales propiedades del etil metil éter (C3H8O) son: Masa molar: 60,09 g/mol. Densidad: 0,72 g/cm 3. Punto de fusión: -139 ºC. Punto de ebullición: 7 ºC. Riesgos. El metoxietano es un compuesto altamente peligroso y su manipulación y uso puede desencadenar en graves efectos para la salud. Una exposición prolongada puede provocar:
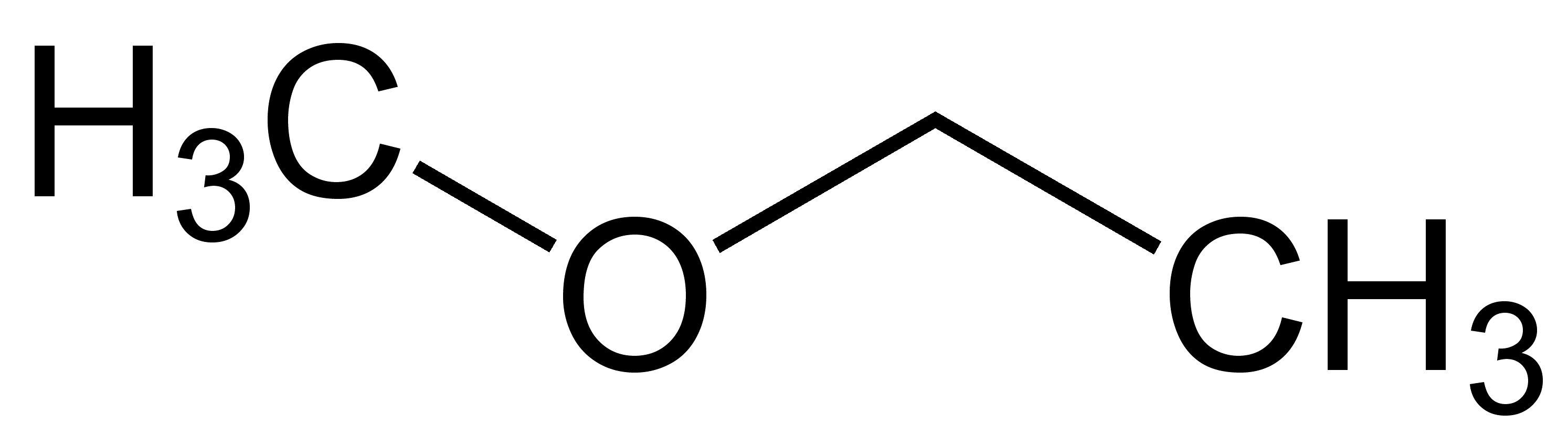
Ethylmethylether
Ethers, such as ETHYL METHYL ETHER, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond.

Ethyl_methyl_ether.svg NOIC
An ester of a carboxylic acid.R stands for any group (typically hydrogen or organyl) and R ′ stands for any organyl group.. In chemistry, an ester is a compound derived from an acid (organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group (−OH) of that acid is replaced by an organyl group (−R).Analogues derived from oxygen replaced by other chalcogens.

Nomenclatura orgánica Nomenclatura de los éteres
Methyl tert-butyl ether (MTBE), also known as tert-butyl methyl ether, is an organic compound with a structural formula (CH 3) 3 COCH 3.MTBE is a volatile, flammable, and colorless liquid that is sparingly soluble in water. Primarily used as a fuel additive, MTBE is blended into gasoline to increase its octane rating and knock resistance, and reduce unwanted emissions.

éter, Metil éter Tertbutyl, Butilo Grupo png transparente grátis
Methoxyethane, also known as ethyl methyl ether, is a colorless gaseous ether with the formula CH3OCH2CH3. Unlike the related dimethyl ether and diethyl ether, which are widely used and studied, this mixed alkyl ether has no current applications. It is a structural isomer of isopropyl alcohol. Its utility as an anesthetic [3] and solvent [4.
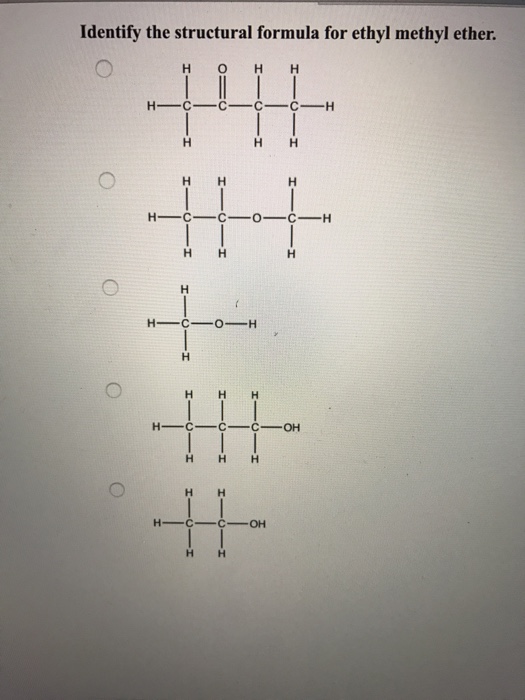
Solved Identify the structural formula for ethyl methyl
88.15 g/mol. Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates. Create: 2005-03-27. Modify: 2024-02-17. Description. Ethyl isopropyl ether is a natural product found in Mangifera indica with data available.

C3H8O Lewis Structure How to Draw the Lewis Structure for C3H8O (ethylmethyl ether) YouTube
NIOSH Method 1610: A gas chromatographic method for the analysis of ethyl ether, consists of a stainless steel column, 1.2 m x 6 mm OD, packed with Porapak Q (50/80 mesh), with hydrogen-air flame ionization detection, and nitrogen as the carrier gas at a flow rate of 30 ml/min, is a NIOSH approved method. A sample injection volume of 5 ul is suggested, the column temperature is 175 °C, the.

éter, Grupo Etilo, Etil Metil Celulosa imagen png imagen transparente descarga gratuita
Etil metil eter ya da metoksietan ilacımsı kokuya sahip renksiz bir gazdır. Yapısal formülü CH 3 OC 2 H 5 olan asimetrik bir eterdir. Erime noktası -113 °C olup 7.4 °C'de kaynar. Fazla yanıcıdır ve bilinen bir kullanım alanı yoktur. Kimya ile ilgili bu madde taslak seviyesindedir.