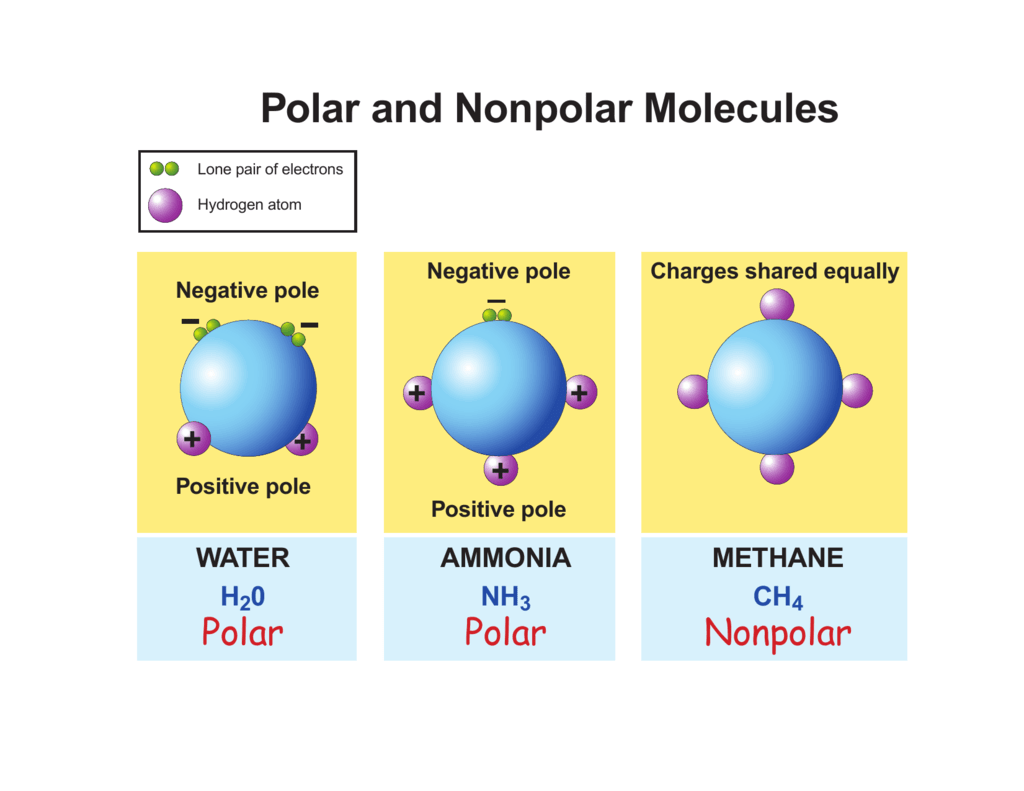
Polar and Nonpolar Molecules
View detailed information about property 4317 Polar Way Unit 24, Riverbank, CA 95367 including listing details, property photos, school and neighborhood data, and much more.
Simple Question Do all polar molecules have a dipole moment? r/chemhelp
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar. Chlorine is a highly reactive element and.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding, Covalent Bonding
The whole community pigments and lipids have been examined during a 5-year period in two commercial solar salterns located in the United States and in Israel. There were significant differences in the complexity of the lipid and pigment patterns within the California saltern system, and these differ.

Polar and Nonpolar Molecules JourneyilSalinas
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.

Cl2 lewis structure, Molecular shape, Polar or NonPolar, Dot diagram
Far West Laboratories, Inc. 6602 2nd Street. Riverbank, CA 95367. Phone: (209) 869-9260. Certificate No. Expiration Date. 1310 2/28/2022. *As of 1/1/2020, this list supersedes all previous lists for this certificate number. Customers: Please verify the current accreditation standing with the State.

Polar Vs Nonpolar Ciencias exatas, Ciência química, Físicoquímica
Electronegativity is a dimensionless number; the greater the electronegativity value, the greater the attraction for shared electrons. Figure 13.5.1 13.5. 1: Electronegativities of the elements. Electronegativities are used to determine the polarity of covalent bonds. An interactive version of this table may be found here .
Ikatan Kovalen Polar Dan Nonpolar Beserta Contoh Ikatannya Rumus Kimia Sexiz Pix
When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively.

Polar and Nonpolar Covalent Bonds Characteristics & Differences Still Education
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
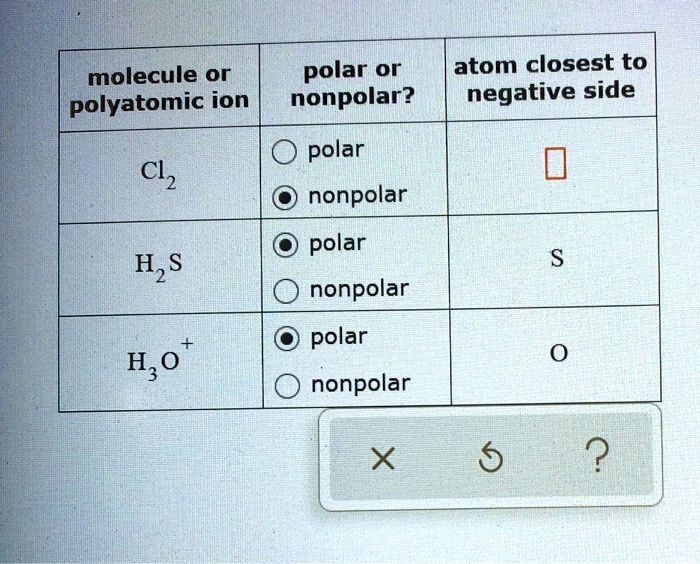
SOLVED molecule or polar or atom closest to polyatomic ion nonpolar? negative side polar Cl2
Hydrogen=2.2, carbon=2.5 and chlorine=3.1. So, electronegativity difference between C-H=0.3 and C-Cl=0.6. It proves that CH2Cl2 is polar but a moderate polar as the difference between their electronegativity is quite small. The total number of valence electrons in the CH2Cl2 molecule is 20. Carbon contains 4 valence electrons and hydrogen has 1.

Cl2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Geometry. To determine if CH 2 Cl 2 (dichloromethane) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. There are 4 + 2 + 2×7 = 20 electrons, and 8 have been used to make four bonds.
Difference Between Polar and Nonpolar Molecules Definition, Formation, Properties, Examples
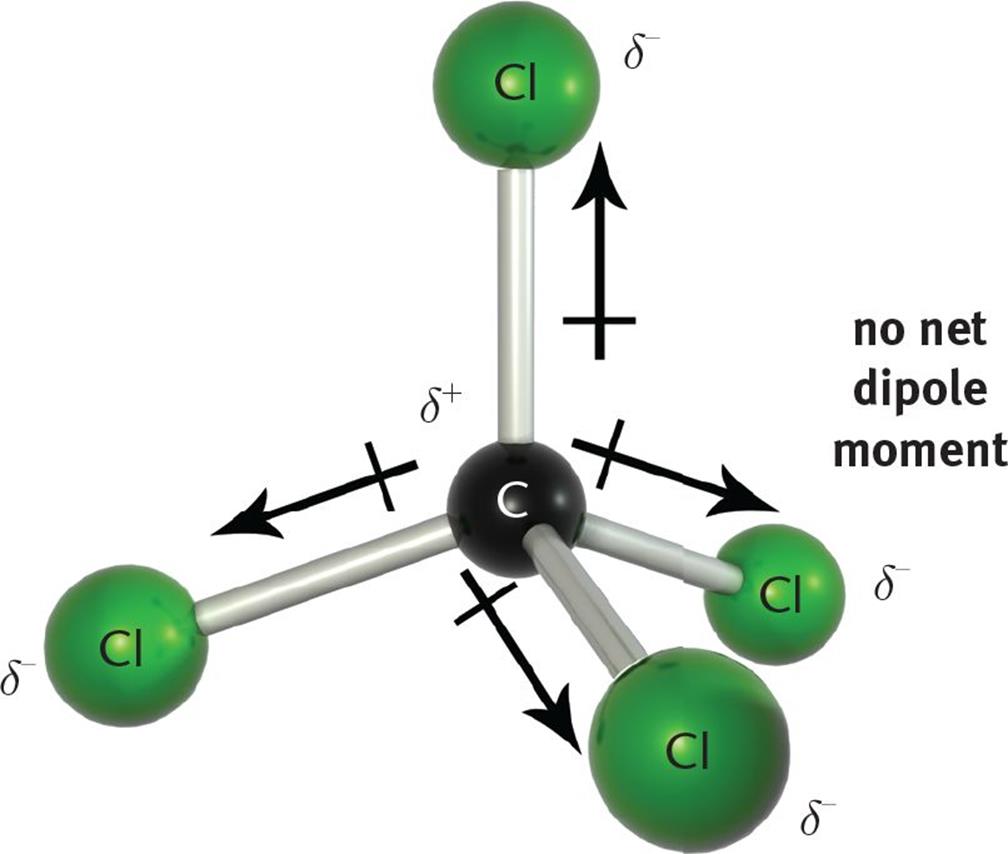
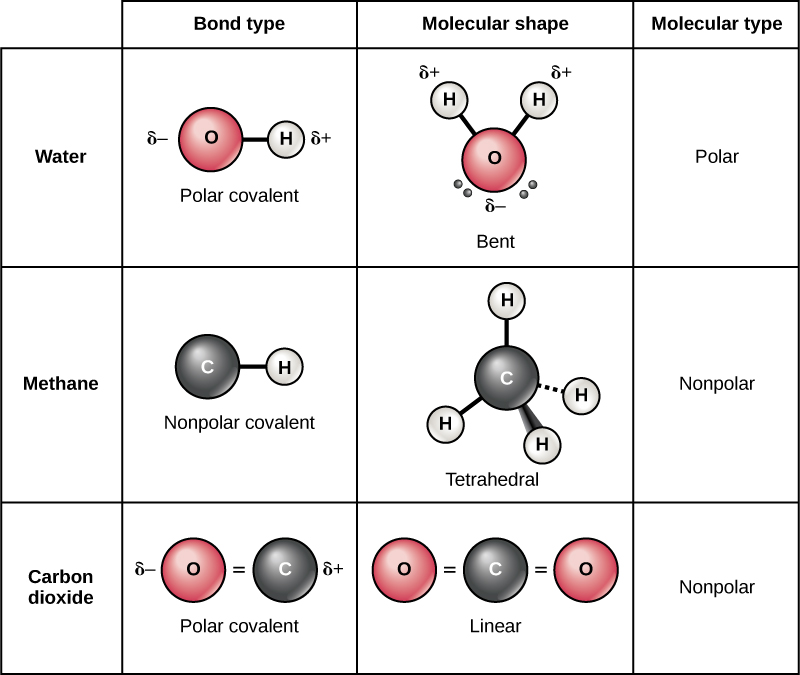
Figure 5.3.7: The molecular geometry of a molecule affects its polarity. In CO 2, the two polar bonds cancel each other out, and the result is a nonpolar molecule. Water is polar because its bent shape means that the two polar bonds do not cancel. Some other molecules are shown below (see figure below).

Elements Lewis Structure/ Sharing of Electrons Type of Bond (polar or nonpolar) Cl2 CHA
The covalent bond formed by the two atoms is said to be non-polar if the electronegativity of both atoms is equal. Note: It is also possible to have polar bonds within a non-polar molecule because the polarity of bonds gets canceled by each other due to symmetric geometrical shape. Few examples of nonpolar molecules are Hexane, CCl4, etc.

MakeTheBrainHappy Is Cl2 Polar or Nonpolar?
Learn to determine if CH2Cl2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.

🔴IKATAN KOVALEN POLAR & NON POLAR, 🔴SENYAWA KOVALEN POLAR DAN SENYAWA KOVALEN NONPOLAR YouTube
Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond. If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent.

Is ClF Polar or Nonpolar (Chlorine Monoluoride) YouTube
The polarity of molecules is related to the polarity of bonds within the molecule, but just having polar bonds is not enough to create a polar molecule. Consider, for example, CCl 4 and CHCl 3. Carbon tetrachloride has 4 fairly polar bonds but they form a regular tetrahedron and the polarity of the individual bonds cancel each other out to.

Is Cl2 Polar or Nonpolar? Techiescientist
Let's dive into it! Cl2 is a NONPOLAR molecule because any two bonding atoms whose electronegativity difference value is less than 0.4 forms a nonpolar bond. Here in Cl2 molecule, both the atoms are Chlorine atoms. Because of this, the electronegativity difference of both the Chlorine atoms (Cl = 3.16) is 0 (i.e 3.16 - 3.16 = 0).