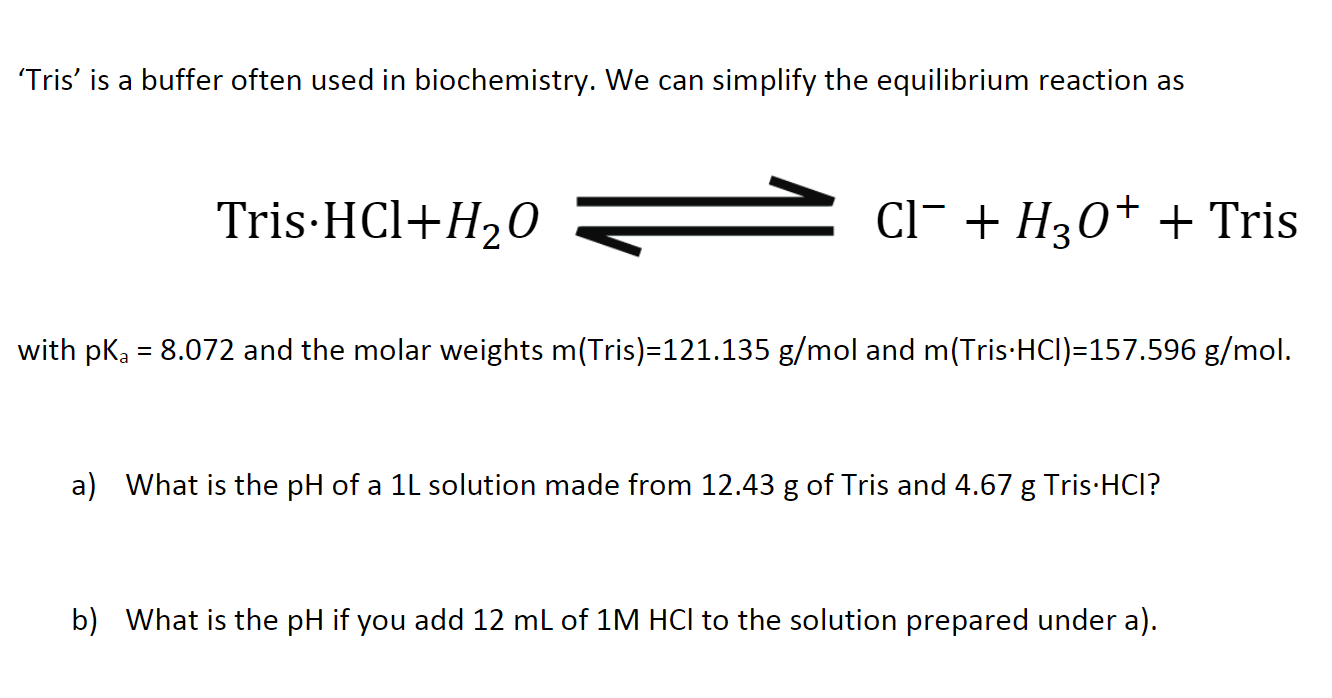
Solved Tris' is a buffer often used in biochemistry. We can
Cara Membuat Solusi Tris Buffer. Larutan penyangga adalah cairan berbasis air yang mengandung asam lemah dan basa konjugasinya. Karena kimia mereka, larutan penyangga dapat menjaga pH (keasaman) pada tingkat hampir konstan bahkan ketika perubahan kimia sedang terjadi.. Campur dalam HCl (misalnya, 1M HCl) sampai pH meter memberi Anda pH yang.
Tris HCl Buffer, pH 10.0 (10x) Delta Microscopies
Mulailah dengan menentukan konsentrasi ( molaritas) dan volume buffer Tris yang ingin Anda buat. Misalnya, larutan buffer Tris yang digunakan untuk saline bervariasi dari 10 hingga 100 mM. Setelah Anda memutuskan apa yang Anda buat, hitung jumlah mol Tris yang dibutuhkan dengan mengalikan konsentrasi molar buffer dengan volume buffer yang dibuat.

Tris Hcl Buffer Ph 9 Recipe Besto Blog
Tris-HCl is a buffer that can be used to control the pH of many solutions, including buffers used in ELISAs, cell and tissue lysis buffers, and buffers for fluorogenic assays. Tris-HCl can be prepared using Tris base (molecular weight: 121.14 g/mol), or Tris-HCl (Tris base which is already combined with HCl in a 1:1 molar ratio, so the.

TrisHCl Buffer 2M, pH 7.5 bioWORLD
Recipe for 100mM Tris Buffer. In a suitable container add target volume of dH20 -10% to allow for pH adjustment. e.g. for 1L Tris, start with 900ml. Add 0.1211g of Tris for each 10ml dH20. e.g. for 900ml add 10.899g Tris. Tris solution will be basic, therefore adjust to target pH 7.0 by addition of HCl. Make up to final target volume with dH20.
Tris Hcl Buffer Ph 9 Recipe Bryont Blog
201-064-4. Tris, also referred to as THAM (Tris (hydroxymethyl)aminomethane), is a white crystalline powder. Tris buffer has a pKa of approximately 8.1 at 25°C making it a good choice for most biological processes in the pH range of 7-9. Compared to other specialized buffers like HEPES, tris powder is a cost-effective and more robust option.

TrisHCl buffer (1mol/L, sterile) (BZ214) Biochemazone™
Tris Hydrochloride is a commonly used biological buffer which is also referred to as Trizma HCl. Tris as a buffer has very broad usage in biological systems such as diagnostics, downstream purification and cell culture. The pKa of Tris is 7.77 which corresponds closely to a physiological pH of 7.36.
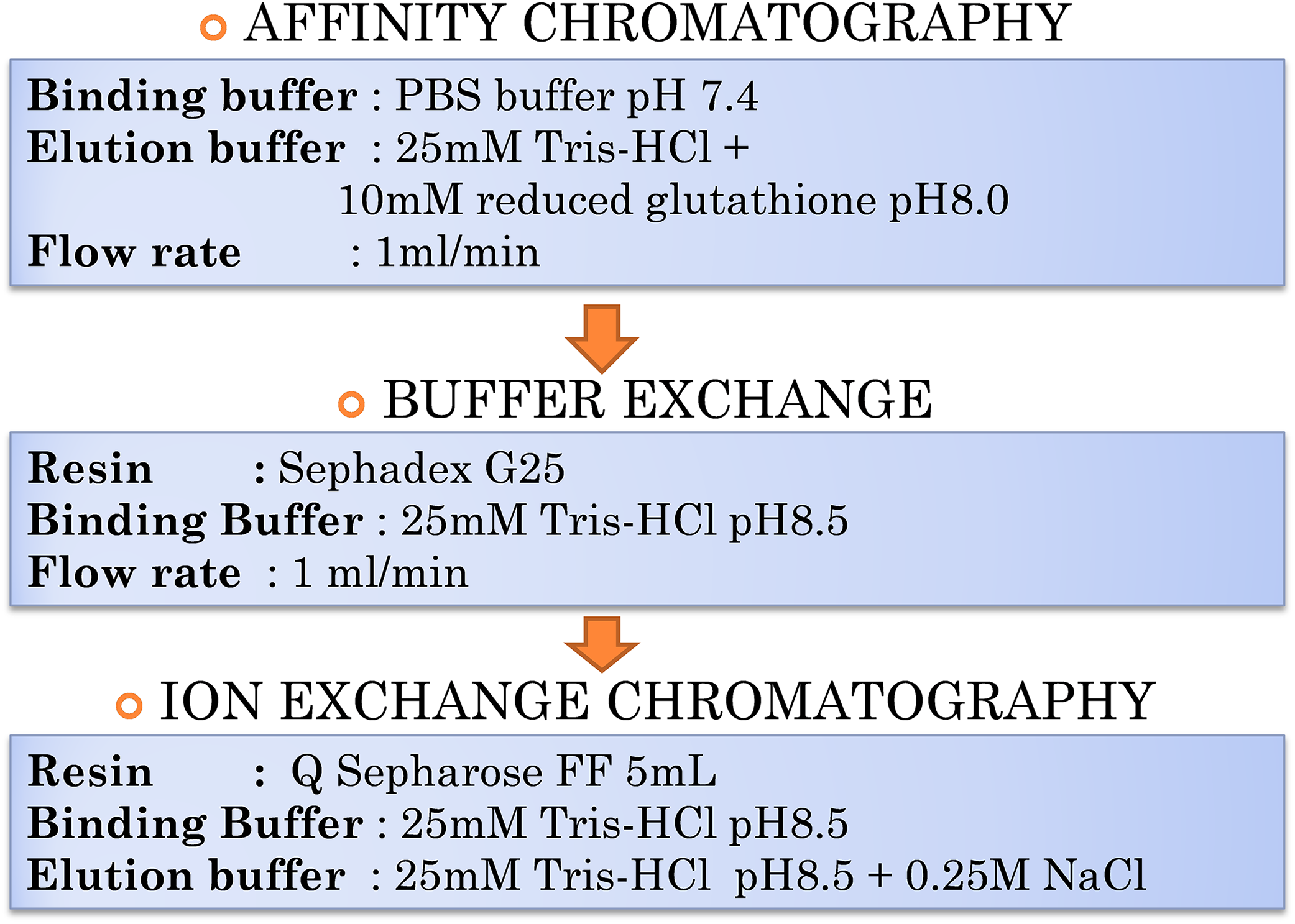
Tris Base And Tris Hcl Buffer Table Table Decorations
The role of Tris-HCl in molecular biology is to control the acidity and osmolarity of a solution. Due to its properties, Tris-HCl is often a component of lysis buffers. The pH of the solution is most commonly made between 7.0 and 8.0. Download the recipe as a PDF. To download the 1 M Tris-HCl recipe as a PDF then click here. 1 M Tris-HCl recipe
TrisHCl Buffer 1M, pH 7.6, Sterile bioWORLD
The pH at this stage is approx. 10.6. When it become homogenous solution, adjust the pH using 6M HCl dropwise until pH 9. Adjust the final volume to 1000 ml (1Lt) and autoclave the solution. The.

Tris Buffer Calculation with Addition of 3 mL of HCl YouTube
Add 15.759 g of Tris-Cl (desired pH) to the solution. Add 2.92 g of EDTA (pH 8) to the solution. TBE buffer is commonly prepared as a 10X concentrated stock. To make the stock solution, dissolve.

Tris Buffer Recipe Table Besto Blog
Buffer and Media Tris-HCl buffer 50 mM pH 8.0: Materials • 4.44 g Tris-HCl • 2.65 g Trisbase • 1 L Double distilled water Procedure 1. Dissolve the Tris-HCl and the Tris base in 1 L of double distilled water. 2. Check pH. If not 8.0, adjust with base or acid. M.A. . Author: Vlad Los Created Date.

Tris Hcl Buffer Recipe Ph 7 5 Bryont Blog
Protocol. Solution A: Dissolve 121.14 g Tris (American Bioanalytical #AB14042) in 800 ml dH 2 O. Adjust pH to 7.0 with the appropriate volume of concentrated HCl. Bring final volume to 1 liter with deionized water. Autoclave and store at room temperature.

Get CH107 100 mM TrisHCl Buffer With pH 7.4 in USA And Canada
Tris-acetate-EDTA (TAE) running buffer and tris-borate-EDTA (TBE) are commonly used buffers for DNA agarose gel electrophoresis that are especially useful in preparative work. 1. Compared to tris-borate-EDTA (TBE) and tris-phosphate-EDTA (TPE) buffers, double-stranded DNA tends to run faster in TAE. However, because TAE has the lowest buffering.

Tris Glycine Buffer Recipe Dandk Organizer
This video explains about how to calibrate pH meter and prepare 1.5 M Tris-HCl buffer. Also explains about precautions need to be taken while preparing buff.

AccuGENE™ 1 M Tris HCl Buffer (주)에코셀
To produce 1M Tris HCl buffer, prepare Tris alkali and hydrochloric acid, dissolve and adjust the pH to the desired value, then make up to volume, filter, package and store.

1M TrisHCl (pH 7.4), 1 L You Do Bio
Tris (mw: 121.14 g/mol) 12.1 g. 0.09988 M. Prepare 800 mL of distilled water in a suitable container. Add 8 g of Sodium chloride to the solution. Add 0.2 g of Potassium Chloride to the solution. Add 12.1 g of Tris to the solution. Adjust the pH to 7.4 with HCl. Add distilled water until the volume is 1 L.
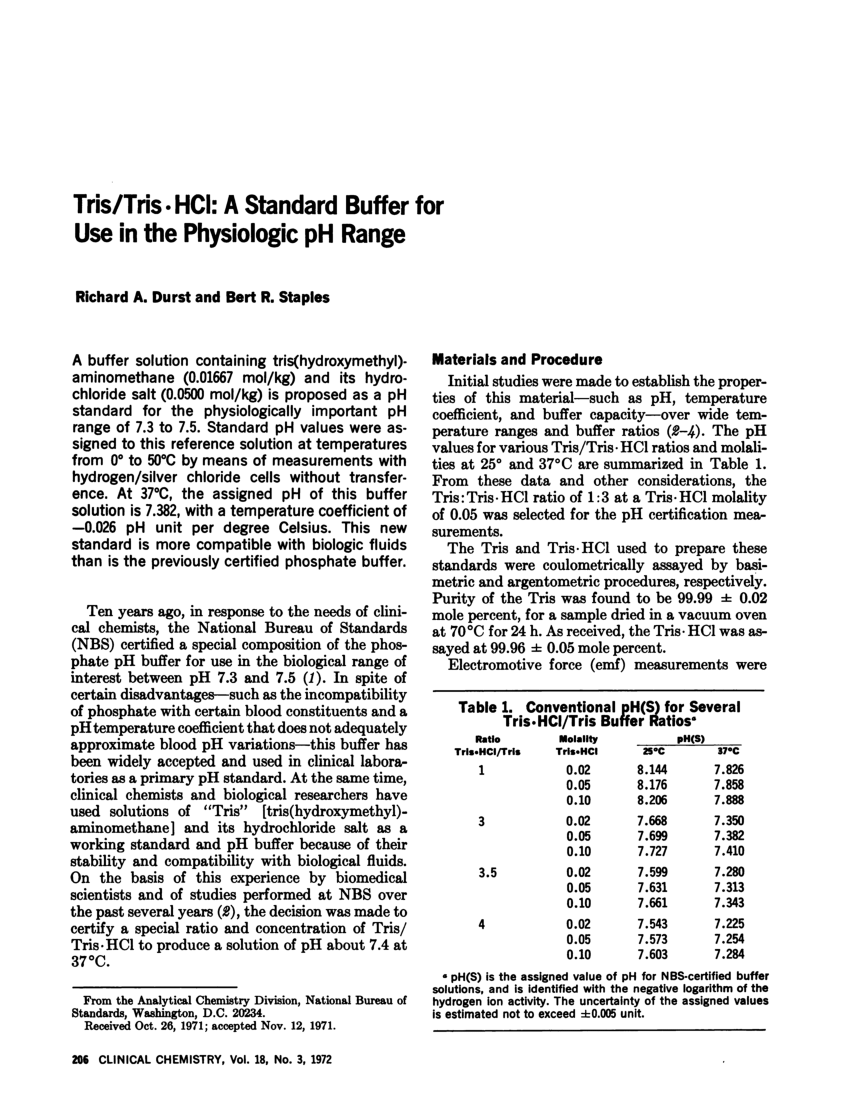
Tris Hcl Buffer Recipe Bryont Blog
Dissolve the Tris into the distilled deionized water, 1/3 to 1/2 of your desired final volume. 4. Mix in HCl (e.g., 1M HCl) until the pH meter gives you the desired pH for your Tris buffer.