SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
Usually, the shape and the molecular geometry of the molecule can vary due to the presence of lone pairs, electronegativity and other related factors. As far as SF2 lewis structure is concerned the central sulphur atom has 2 lone pairs of electrons and 2 bond pairs with fluorine atoms. Due to the presence of lone pairs on sulphur atoms and the.

Sf2 molecular geometry zeseoseouc
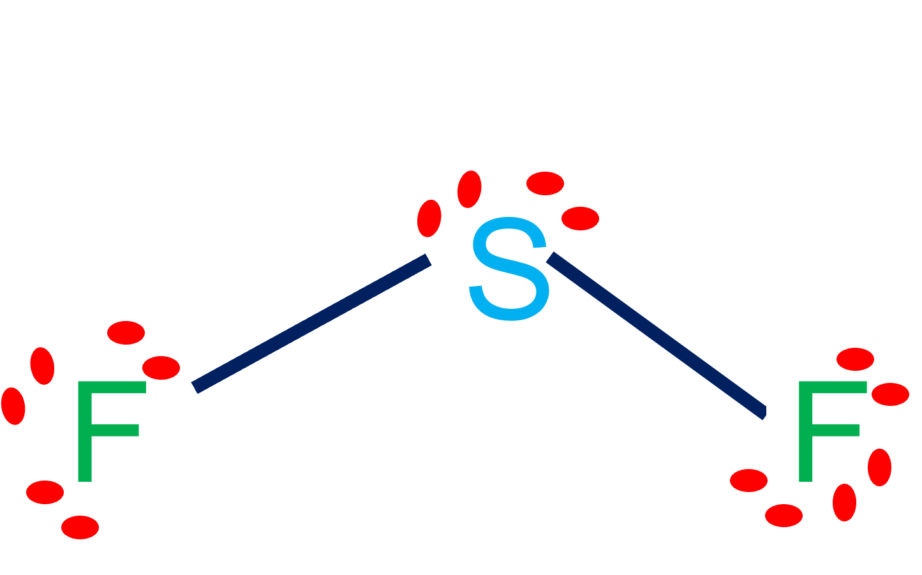
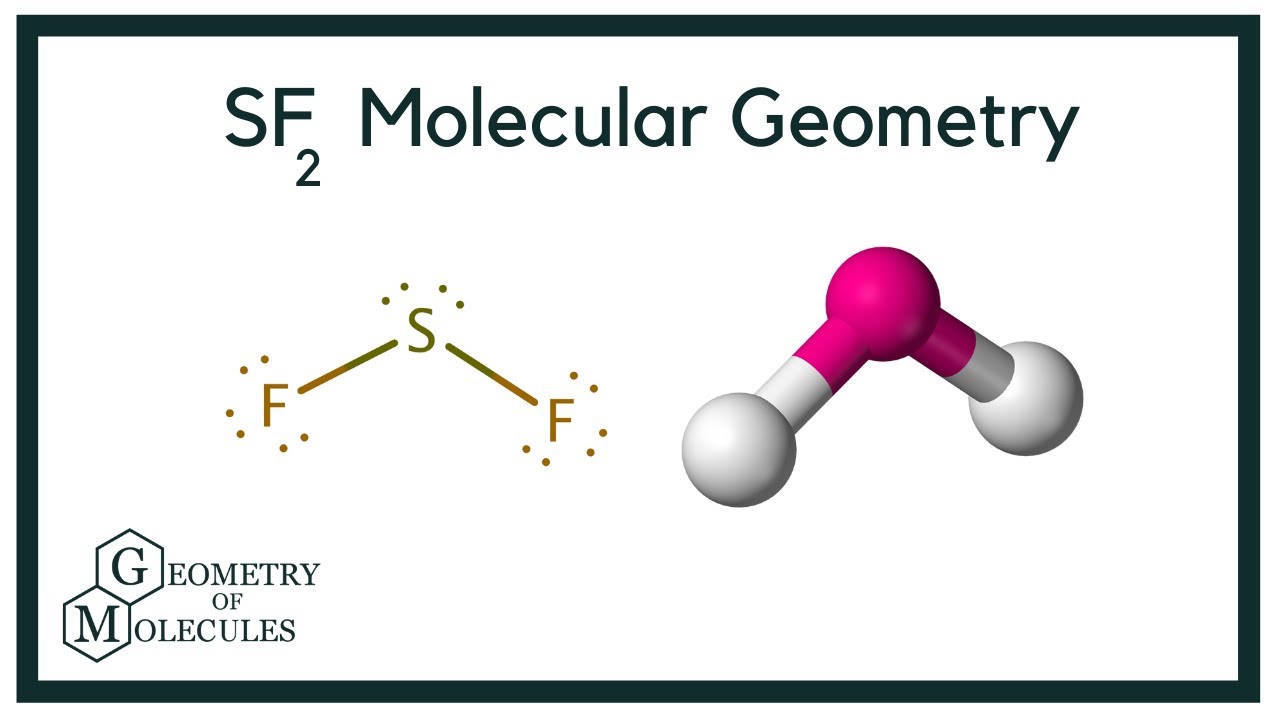
SF2 molecular geometry is bent. SF2 molecule is polar in nature. In the SF2 lewis structure, there is a single bond between sulfur and two fluorine atoms. SF2 Hybridization. The electronic configuration of Sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. First, the electrons are filled in 1s, then in 2s, and so on.
Sf2 Lewis Structure Shape I also go over formal charge, hybridization
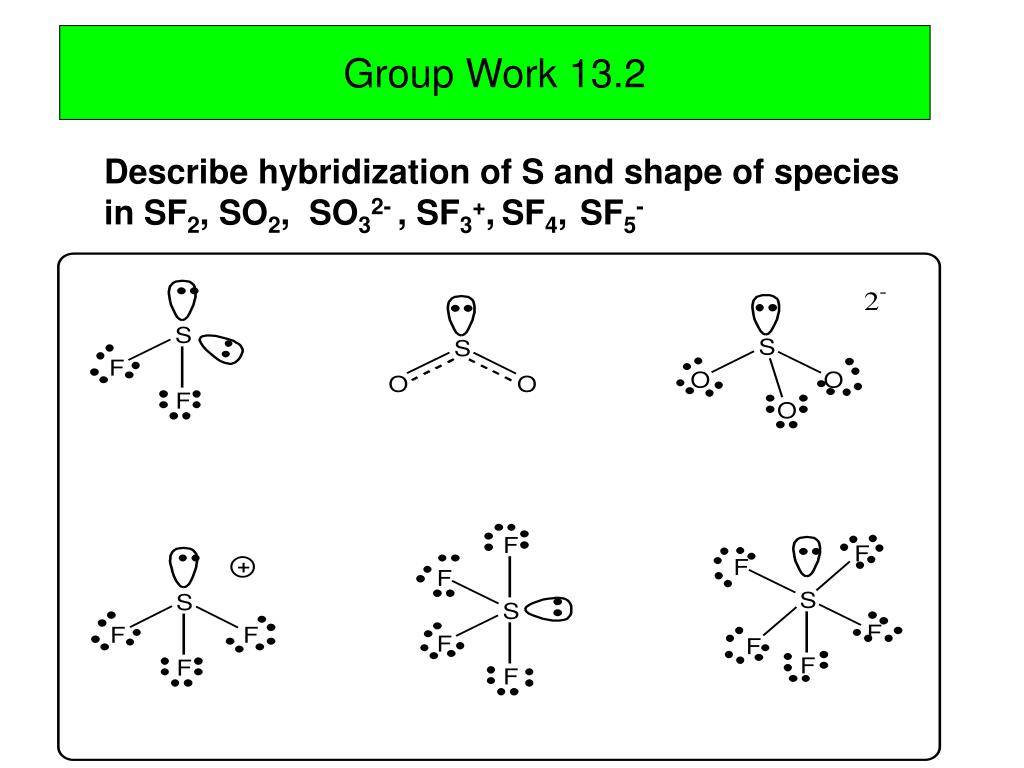
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.
Top Sf2 Molecular Geometry Bond Angle Full GM
SF2 Molecular Geometry. The molecular geometry of the molecule depends on the Lewis structure and the arrangement of valence electrons in the structure. The sulfur atom has two bonding pairs of electrons and two nonbonding pairs of electrons that represent the VSEPR notion of AX2E2, which corresponds to an angular/non-linear or bent molecular.
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
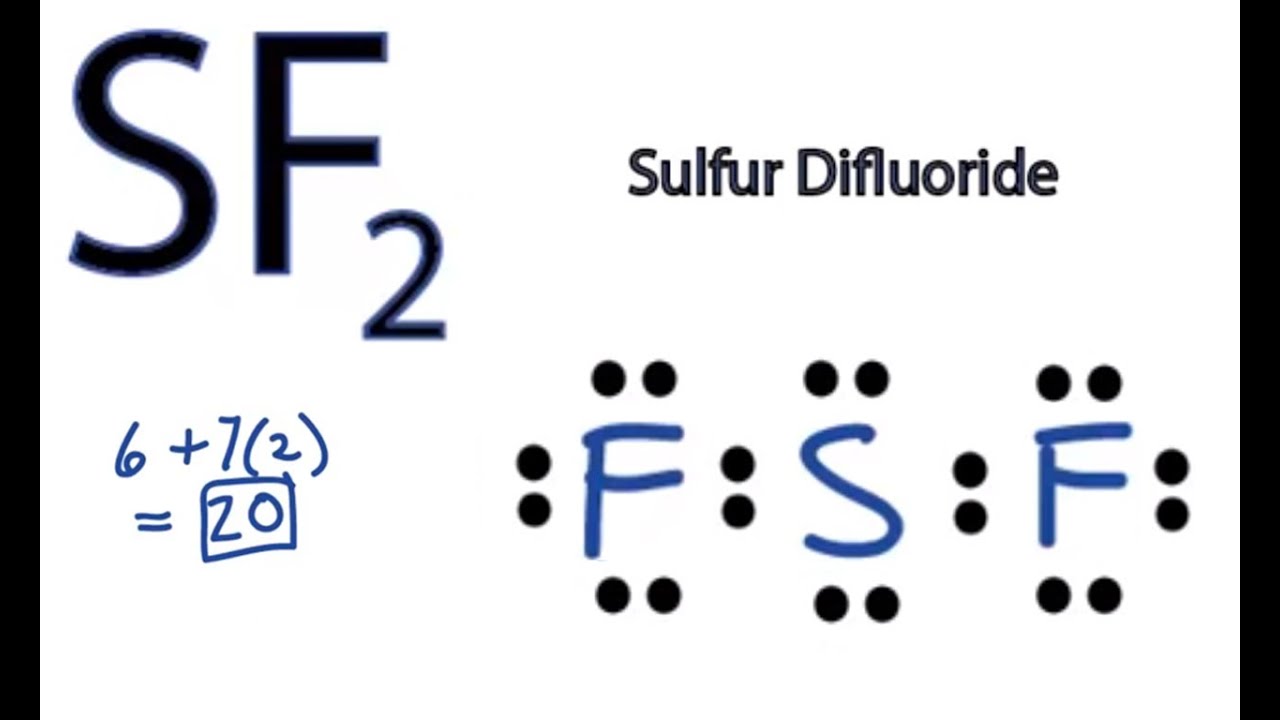
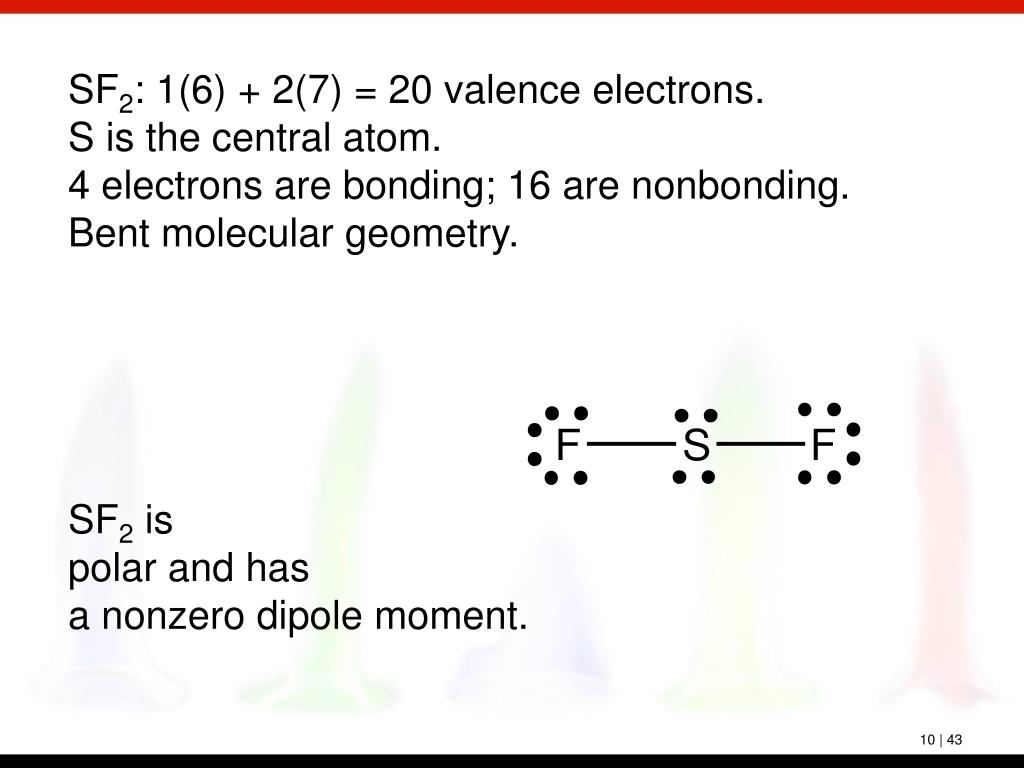
Let's do the SF2 Lewis structure. Sulfur has 6 valence electrons. Fluorine has 7, but we have two of them. Six plus 14 equals 20 total valence electrons. We'll put Sulfur at the center and the Fluorines on either side. Put a pair of electrons between atoms to form chemical bonds. We've used four; and then on the outside, 6, 8, 10, 12, 14, 16.

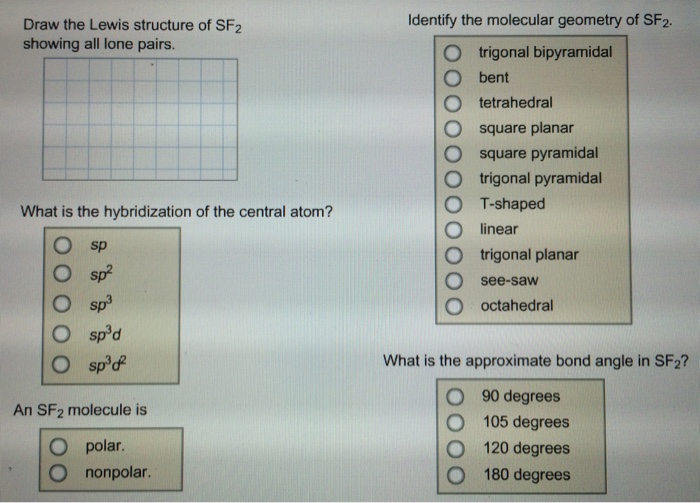
Draw the Lewis structure of SF2, showing all lone pairs. Identify the
The electron geometry for SF2 is tetrahedral. Because 4 electrons which make 2 lone pairs around a sulfur atom are arranged in a tetrahedral geometry. The bond angle of SF2 is around 98º. The lewis structure of SF2 has 4 bonding electrons and 16 nonbonding electrons. The hybridization for SF2 is Sp 3.

SF2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
Hello Guys! Welcome back to our channel and in today's video we are going to share a step-by-step method to determine the molecular geometry of the given mol.
Solved Draw the Lewis structure of SF2 showing all lone
SF2. Skip to main content. General Chemistry.. Molecular Shapes & Valence Bond Theory 1h 22m. Worksheet. Valence Shell Electron Pair Repulsion Theory 5m. Equatorial and Axial Positions 9m. Electron Geometry 7m. Molecular Geometry 12m. Bond Angles 7m. Hybridization 7m. Molecular Orbital Theory 11m. MO Theory: Homonuclear Diatomic Molecules 6m.

SF2 Lewis structure, Molecular geometry, Hybridization, Polar or nonpolar
SF2 consists of one sulfur (S) atom and two fluorine (F) atoms. Sulfur is in Group 16 of the periodic table and has six valence electrons, while fluorine is in Group 17 and has seven valence electrons. Drawing the Lewis Structure of SF2. 1. Calculate the Total Number of Valence Electrons

Is SF2 Polar or Nonpolar? Techiescientist
Geometry. SF2 Geometry and Hybridization. Sulfur is the central atom. There are 6 + 2×7 = 20 electrons, and 4 of them are used to make 2 bonds. The two fluorines take 6 lone pairs, and the remaining four electrons go to the sulfur: The central atom has a steric number of 4 - two atoms and two lone pairs. The electron geometry, therefore, is.
SF2 Lewis Structure How to Draw the Lewis Structure for SF2 YouTube
The geometry of BCl3 BCl 3 is also given in Figure 7.2: it is trigonal planar, with all four atoms lying in the same plane, and all Cl−B−Cl Cl − B − Cl bond angles equal to 120o 120 o. The three Cl Cl atoms form an equilateral triangle. The Boron atom has only three pairs of valence shell electrons in BCl3 BCl 3.
SF2 Molecular Geometry, Bond Angles & Electron Geometry (Sulfur
Here's how you can easily draw the SF 2 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let's take a closer look at each step mentioned above.

Sf2 Lewis Structure Shape I also go over formal charge, hybridization
The center sulfur atom of SF2 has two lone pairs of electrons, resulting in tetrahedral SF2 electron geometry. However, the molecular geometry of SF2 looks tetrahedral or v-shaped and has two lone pairs of electrons on the sulfur of the SF2 geometry. It's the SF2 molecule's symmetrical geometry. As a result, the SF2 molecule is polar.
.jpg)
Sf2 Lewis Structure Shape I also go over formal charge, hybridization
SF2 molecular geometry. The . SF2 molecule has a nonzero net dipole moment. The electron dot structure of the . SF2 molecule is also known as the . SF2 Lewis structure. It determines the number of outermost valence electrons as well as the electrons engaged in the . SF2 molecule's bond formation. The outermost valence electrons of the
Geometry Sf2 Lewis Structure Identify the molecular geometry of sf2
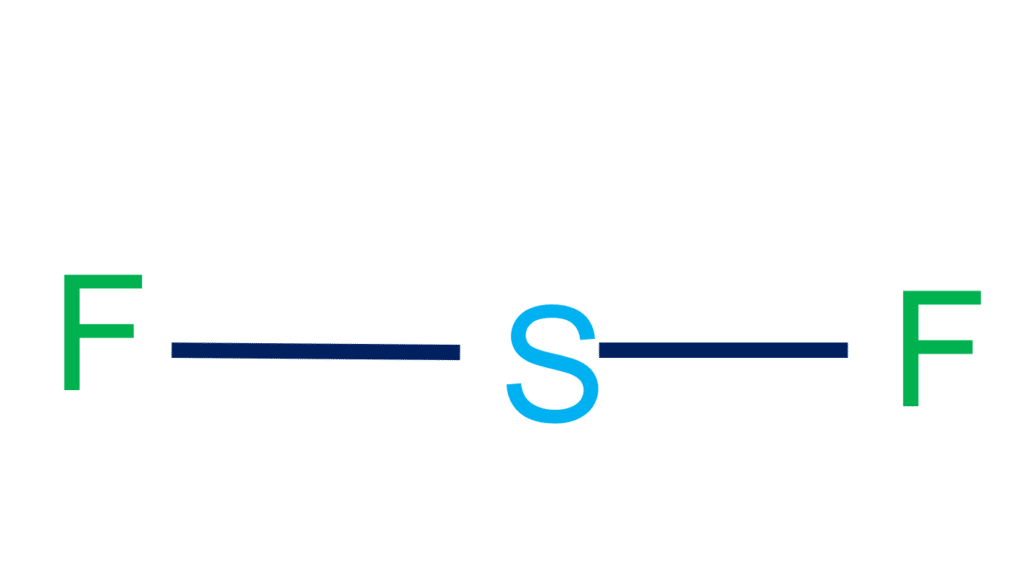
Below is the image of the geometry of SF2. Lewis structure of SF2. Lewis structure of sulfur difluoride is similar to other halogen compounds, sulphur has 6 electrons in the valence shell. Fluorine has 7 valence electrons. Fluorine shares two electrons from sulfur and completes its octet.. The molecular mass of the SF2 molecule is 70.062 g/mol.

SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Chemistry questions and answers. Identify the molecular geometry of SF2 Draw the Lewis structure of SF2 showing all lone pairs O square pyramidal O trigonal bipyramidal O trigonal planar O octahedra O square planar O T-shaped O tetrahedra O trigonal pyramidal O bent What is the hybridization of the central atom? What is the approximate bond.