
Rebalance Soft Academic Cavesson Etsy Horses, Etsy, Bitless bridle
Equine Protozoal Myeloencephalitis (EPM) is an infectious disease of the horse's central nervous system.. It is one of the most difficult diseases for veterinarians to diagnose because it often mimics other conditions and has a wide range of symptoms that affect multiple parts of the horse's body.. Horses affected by EPM may show a lack of coordination in their movements, usually worse on.

Equine Bowen Therapy Does Bowen Therapy promote relaxation? This
EPM is one of the more common neurological infectious diseases of horses in North America, but the disease may be seen in Europe in imported horses. Most cases are caused by Sarcocystis neurona and less commonly Neospora hughesi, both protozoan apicomplexan parasites. ReBalance ® Antiprotozoal Oral Suspension is indicated for the treatment of.

Barastoc Rebalance Protein & Mineral Horse Block
The safety of ReBalance ® Antiprotozoal Oral Suspension with concomitant therapies in horses has not been evaluated. Adverse Reactions. Seventy-five horses (37 horses in the 1X group; 38 horses in the 2X group) that were treated with test article for at least 90 days were evaluated for adverse reactions. Bone marrow suppression:

The point of the lateral work is to rebalance, engage, and strengthen
EPM is a nervous system disease developed by horses when ingesting contaminated feed or water with the protozoa sarcocystis neurona. Each ml of ReBalance Antiprotozoal oral suspension contains 250 mg of sulfadiazine and 12.5 mg pyrimethamine. The usual treatment regimen varies from 90 to 270 days. Labeled for: horses. Species: horse.
Hobilars & Border Horses image Vanilla Reskin & Rebalance mod for
Published by Clayton Newton on November 28, 2022. ReBalance Antiprotozoal Oral Suspension is for oral use only in horses. The usual duration of treatment is dependent on clinical response, but the usual treatment regimen ranges from 90 to 270 days.

ReBalance® Oral Suspension Santa Cruz Animal Health
ReBalance Antiprotozoal Oral Suspension is indicated for the treatment of horses with equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona. ReBalance Antiprotozoal Oral Suspension is to be administered at a dose of 20 mg/kg sulfadiazine and 1 mg/kg pyrimethamine daily or 4 mL of ReBalance Antiprotozoal Oral Suspension per 110.

ReBalance® Antiprotozoal Oral Suspension PRN Pharmacal
Great Low Price. ReBalance Antiprotozoal Oral Suspension is indicated for the treatment of horses with equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona. Each ml of ReBalance Antiprotozoal Oral Suspension contains 250 mg sulfadiazine and 12.5 mg pyrimethamine. Give 4 ml per 110 lbs. body weight orally once per day.

Imagine your horse is a car skidding on ice and you must turn in the
When administered under labeled conditions, ReBalance is an FDA-approved, safe and effective treatment for horses with equine protozoal myeloencephalitis ( EPM ) caused by Sarcocystis neurona. Product Information Manufactured for PRN Pharmacal. Active Ingredients Sulfadiazine 250mg/mL, Pyrimethamine 12.5mg/mL Direction
REBALANCE Antiprotozoal Oral Suspension for Horses, 1qt
The safe use of ReBalance Antiprotozoal Oral Suspension in horses used for breeding purposes, during pregnancy, or in lactating mares has not been evaluated. The safety of ReBalance with concomitant therapies in horses has not been evaluated. ReBalance is not for use in horses with known hypersensitivity to sulfonamide drugs or pyrimethamine.

REBALANCE Antiprotozoal Oral Suspension for Horses, 1qt
Dosage and Administration : ReBalance Antiprotozoal Oral Suspension is to be administered at a dose of 20 mg/kg sulfadiazine and 1 mg/kg pyrimethamine daily or 4 mL of ReBalance Antiprotozoal Oral Suspension per 110 lb (50 kg) of body weight once per day. The duration of treatment is dependent upon clinical response, but the usual treatment.
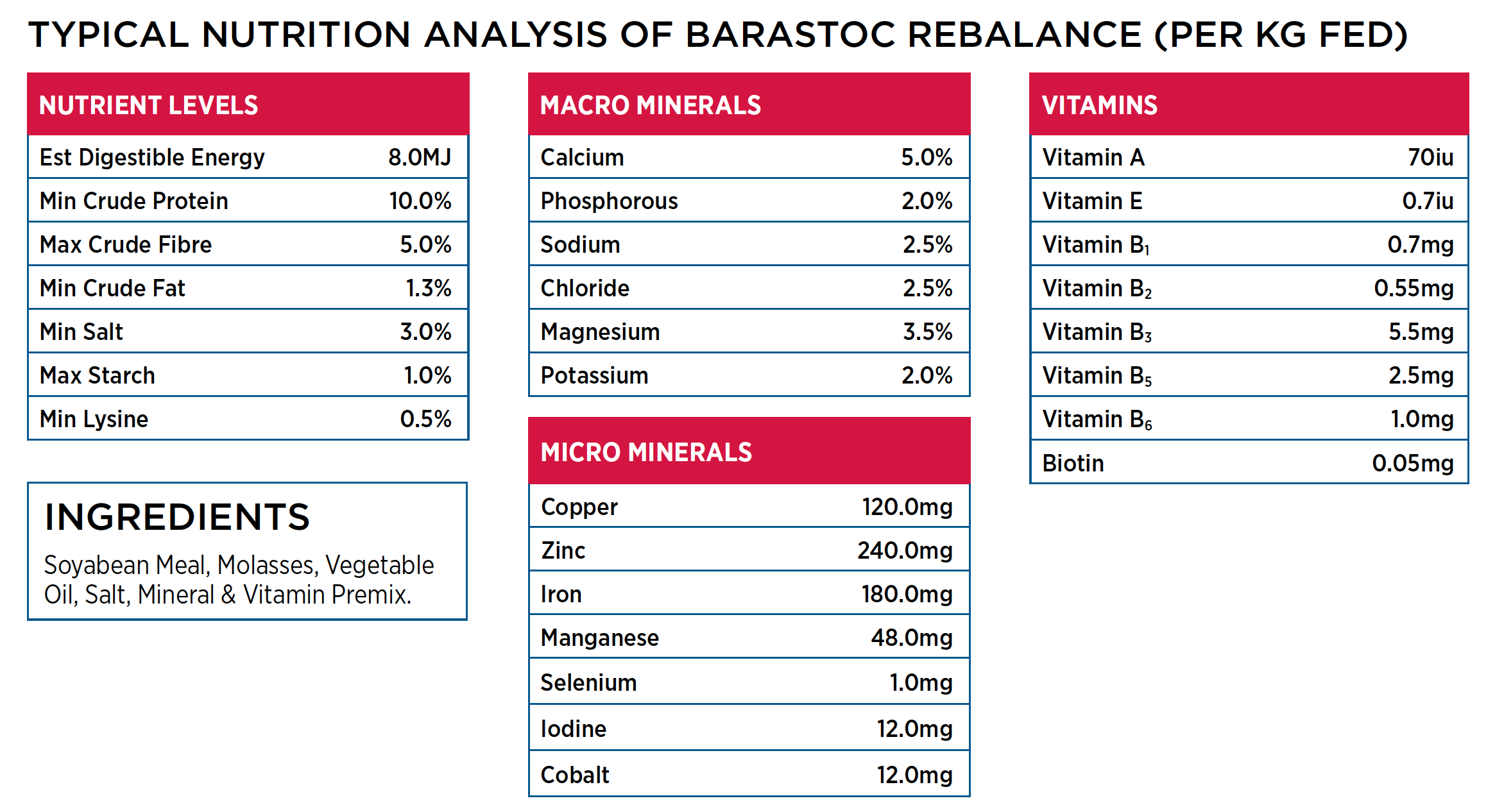
Barastoc Rebalance Horse Block
ReBalance is an FDA-approved, safe and effective treatment for horses with equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona. The active ingredients (Sulfadiazine 250mg/ml and pyrimethamine 12.5mg/ml) inhibit folic acid synthesis at two different sites, in the same synthetic pathway. 32oz bottle.

REBALANCE your (Shire)horse YouTube
Prior to treatment with ReBalance ® Antiprotozoal Oral Suspension, EPM should be distinguished from other diseases that may cause ataxia in horses. Injuries or lameness may also complicate the evaluation of an animal with EPM. In most instances, ataxia due to EPM is asymmetrical and affects the front and/or the hind limbs.

ReBalance® Oral Suspension Santa Cruz Animal Health
Updated May 8, 2023. Equine Protozoal Myeloencephalitis (EPM) is a disease of the central nervous system (brain and spinal cord) caused by the protozoa Sarcocystis neurona. EPM is transmitted by the opossum through intermediate hosts, also known as carriers, which may include birds, cats, and other animals.

Could your energetic imbalance be affecting your horse, or vice versa
ReBalance Antiprotozoal Oral Suspension is indicated for the treatment of horses with equine protozoal myeloencephalitis (EPM) caused by Sarcocystis neurona. Each ml of ReBalance Antiprotozoal Oral Suspension contains 250 mg sulfadiazine and 12.5 mg pyrimethamine. Give 4 ml per 110 lbs. body weight orally once per day. The usual treatment regimen ranges from 90 to 270 days.

Rebalance Golden Pulse Clinic
Typical treatment regimens last between 90 and 270 days. Use a suitable syringe to administer orally a minimum of 1 hour before feeding grain or hay. Insert the syringe nozzle through the interdental space. Depress the plunger to deposit onto the back of the tongue. Follow your veterinarian's directions for use.

Holistic Healing Therapeutic Treatment for Horses Big Dee's Tack
Forty-eight horses were assigned to the 20 mg sulfadiazine/kg and 1 mg pyrimethamine/kg dose of REBALANCE Antiprotozoal Oral Suspension (1X treatment group). The final database consisted of 26 horses treated at the 1X dose, the other 22 horses in the 1X group failing to complete the study. Day 0 was the day the horse was first administered test