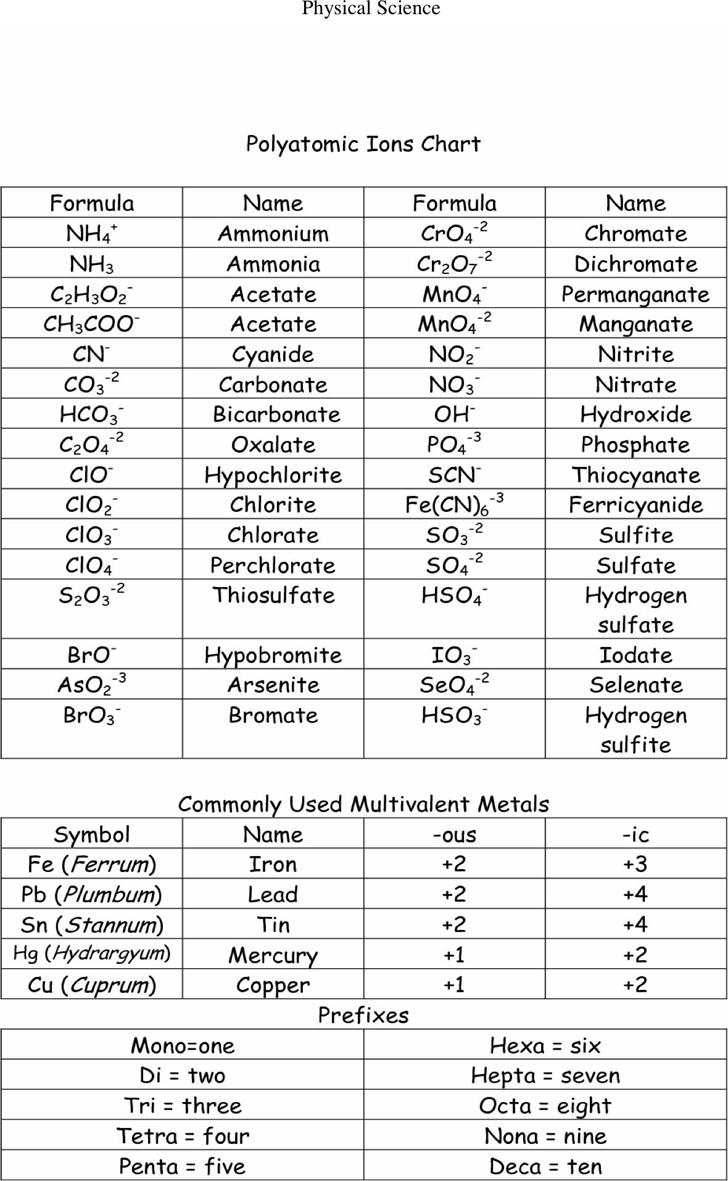
Polyatomic Ions Chart Template Free Download Speedy Template
Table of Polyatomic Ions. There are a number of ions that are not individual atoms but are composed of multiple atoms that are covalently bonded together. However, this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. These polyatomic ions are extremely common in chemistry and thus.
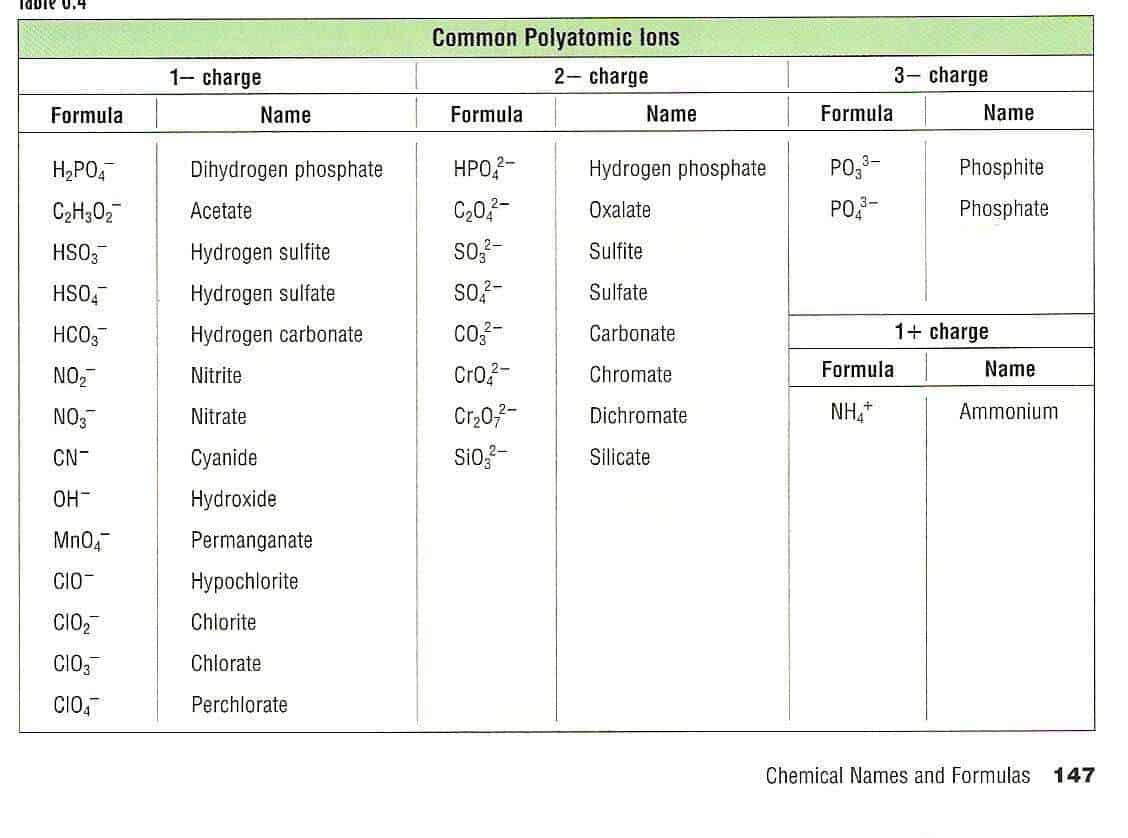
5+ Polyatomic Ion Charts Word Excel Templates
Chemistry library Course: Chemistry library > Unit 1 Lesson 3: Names and formulas of ionic compounds Naming monatomic ions and ionic compounds Common polyatomic ions Polyatomic ions Naming ionic compound with polyvalent ion Worked example: Finding the formula of an ionic compound Predict the charge on monatomic ions Naming ionic compounds
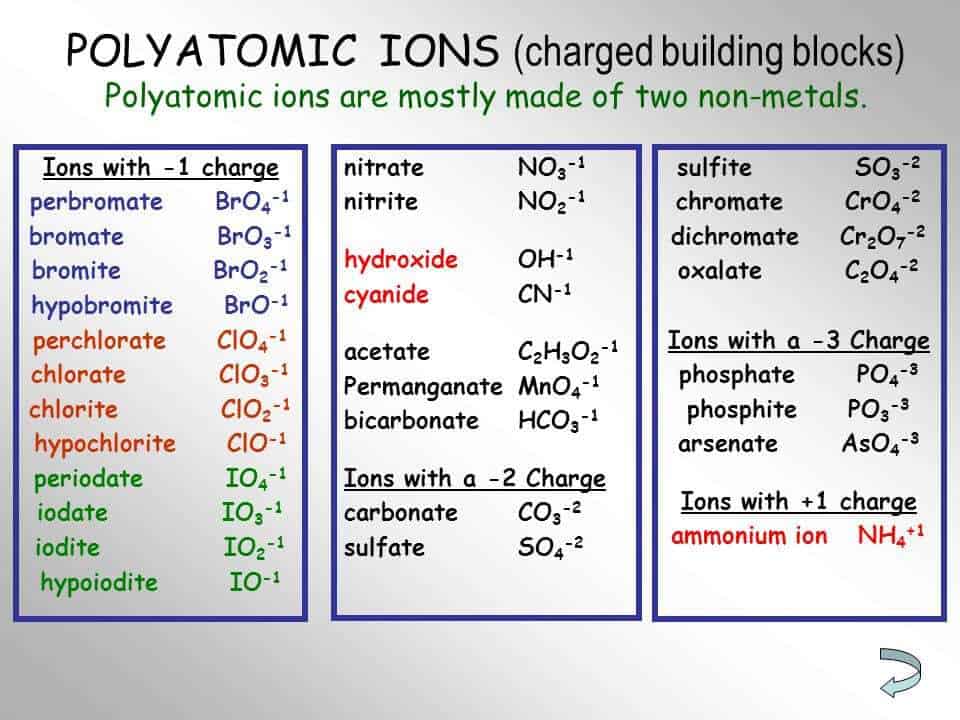
Polyatomic Ion Charts Find Word Templates
Ions made up of more than one atom are known as polyatomic ions. Ions with positive charge are called cations. Ions with negative charge are called anions. List of monatomic ions The ions made of a single atom are called simple ions or monatomic ions. Frequently Asked Questions on Polyatomic ions list Q1 What are 3 examples of polyatomic ions?

Polyatomic Ions Chart 15 Free Templates in PDF, Word, Excel Download
The table shows the names and formulae of some polyatomic ions close polyatomic ion Charged particle consisting of two or more atoms joined together.. Cations Anions
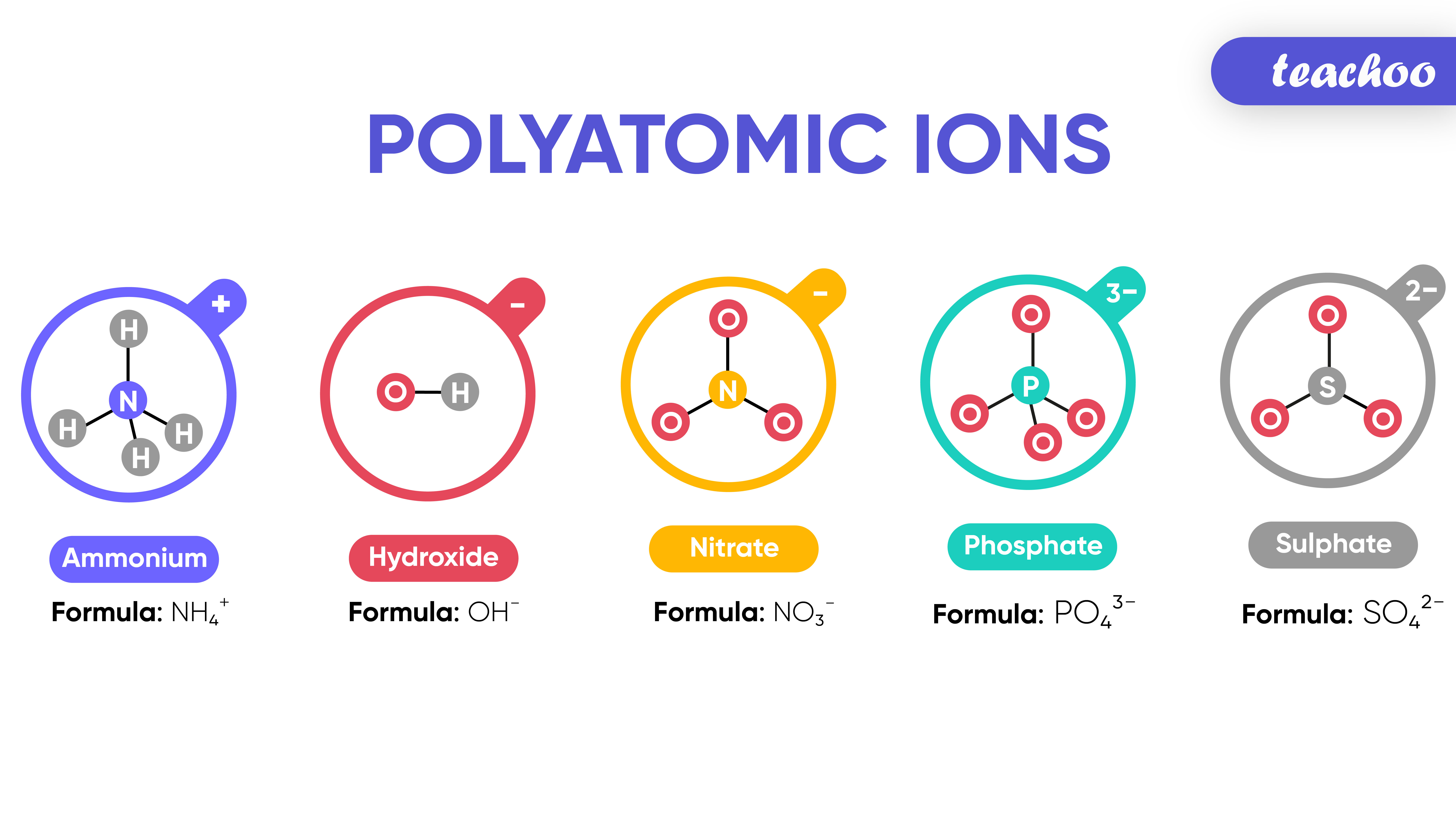
What are Polyatomic Ions? Give Examples Teachoo Concepts
Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. Table 6.3.2 6.3. 2 lists the ion names and ion formulas of the most common polyatomic ions. For example, NO−3 NO 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge.

Periodic Table With Polyatomic Ions Pdf
POSITIVE POLYATOMIC IONS TABLE OF POLYATOMIC IONS H2PO4 - HCO3 - HC2O4 - HSO4 - HS- HSO3 - OH- ClO- IO3 - HPO4 2- NO3 - NO2 - SiO4 4- hydrogen carbonate hydrogen oxalate hydrogen sulfate hydrogen sulfide hydrogen sulfite hydroxide hypochlorite iodate nitrate nitrite orthosilicate monohydrogen phosphate dihydrogen.

Polyatomic Ions Lessons TES
Chemists use the term "polyatomic" to refer to ions that have more than one atom. This is in contrast to so-called "monoatomic" ions that, predictably, only have one ion. Monoatomic ions are more straightforward to understand and there's no memorization necessary, since they're simply ionic forms of the periodic table elements.
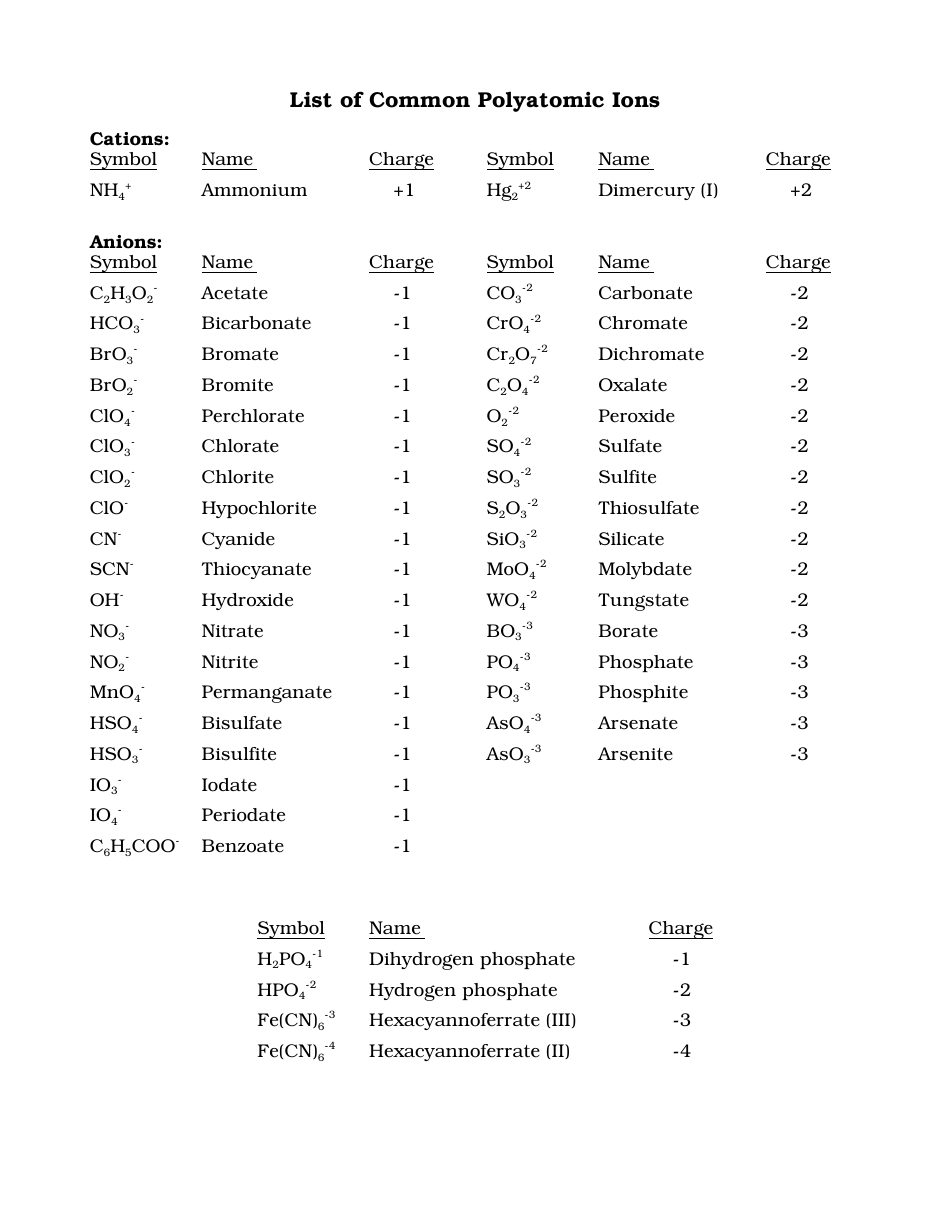
Common Polyatomic Ions Chart Cations, Anions Download Printable PDF
Because these ions contain more than one atom, they are called polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. For example, NO 3 − is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge. Table \(\PageIndex{1}\) lists the most common polyatomic.

Polyatomic Ions Table Free Download
Polyatomic ions. Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. The examples include cations like ammonium ion ( NH+4 NH 4 + ), and hydronium ion ( H3O+ H 3 O + ); and anions like hydroxide ion ( OH− OH − ), and.

Search Results for “Polyatomic Chart” Calendar 2015
Table 1. Common Polyatomic Ions: The nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding. When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of.
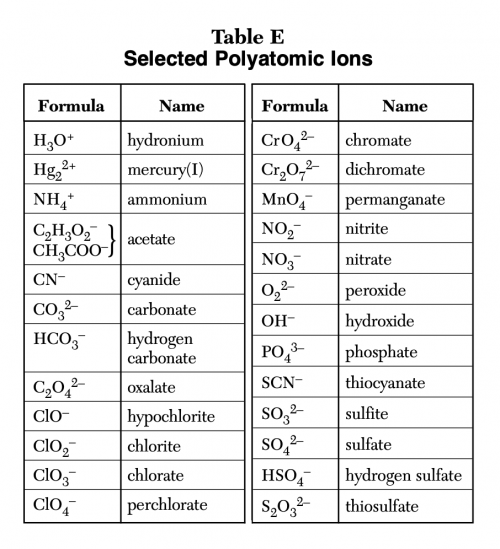
Create a Polyatomic Ions from Table E Tier List TierMaker
Science Intro to polyatomic ions Google Classroom Learn what polyatomic ions are and how they bond. Some ions consist of a single atom with a net charge. They're called monatomic ions. Examples include Na + , O 2 − , and Cl − . Other ions consist of a molecule —a group of atoms covalently bonded together—with a net charge.

Nomenclature of Acids Pathways to Chemistry
A polyatomic ion (also known as a molecular ion) is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. [1] The term molecule may or may not be used to refer to a polyatomic ion, depending on the definition used.
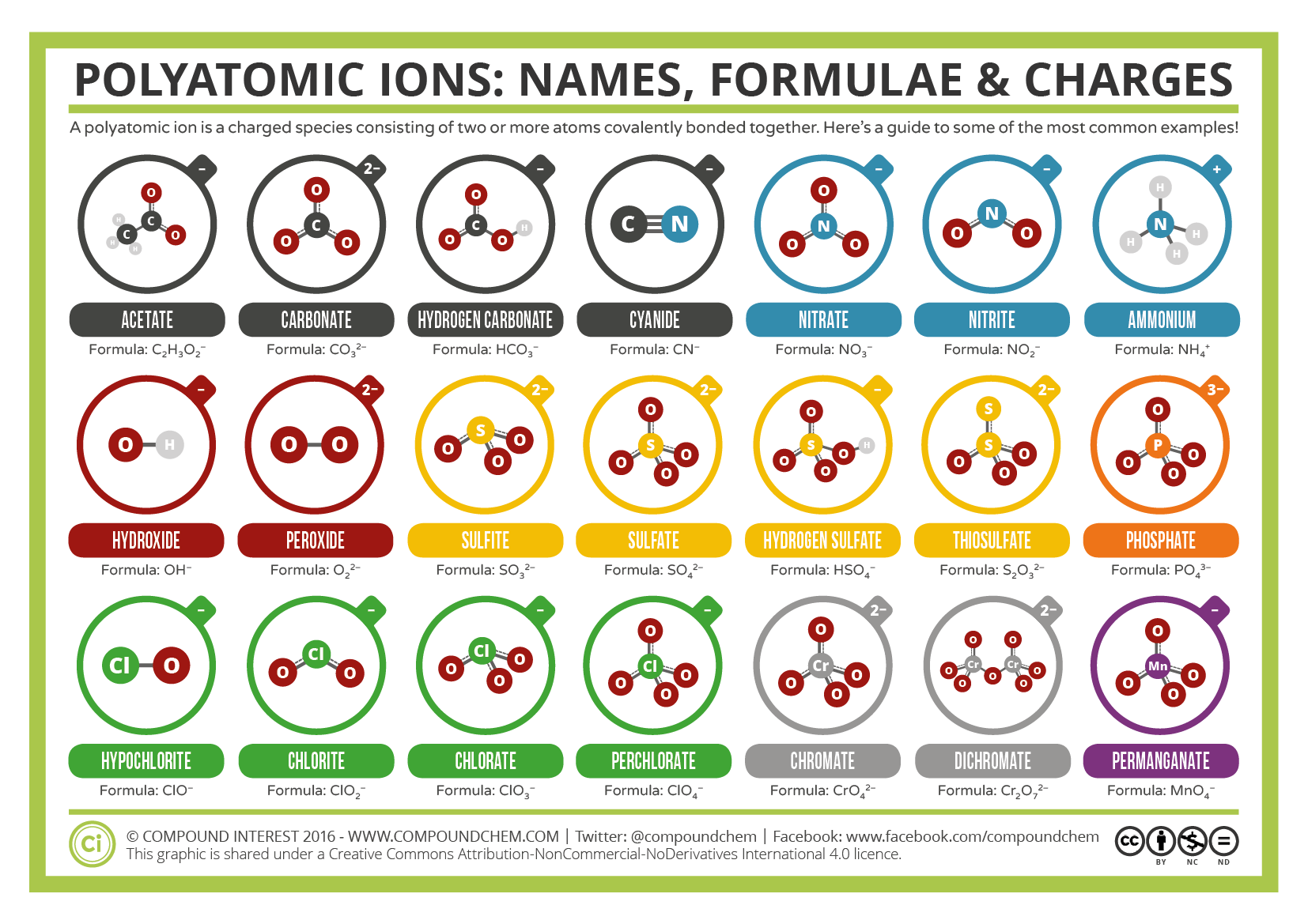
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
Table 6.4a: Common Polyatomic Ions. The naming of ionic compounds that contain polyatomic ions follows the same rules as the naming for other ionic compounds: simply combine the name of the cation and the name of the anion. Do not use numerical prefixes in the name if there is more than one polyatomic ion; the only exception to this is if the.
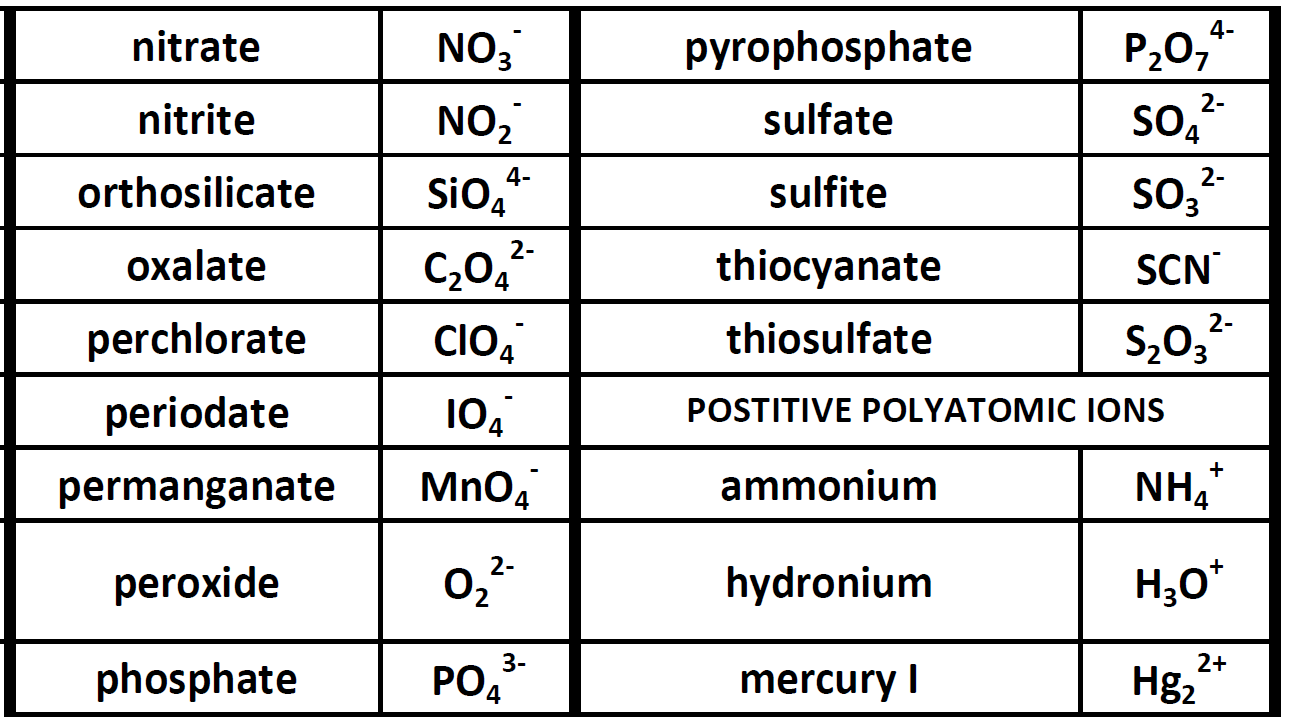
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
7: Chemical Nomenclature

17 best ideas about Polyatomic Ion on Pinterest Chemistry, Chemistry
Polyatomic ions are formed when a group of atoms have a charge. Hydroxide, for example, is formed when oxygen and hydrogen covalently bond but still have a charge of -1. When a polyatomic ion forms an ionic bond with another ion, a polyatomic ionic compound is made. For example, the +1 barium ion can form an ionic bond with the -1 hydroxide ion, to form the Barium Hydroxide (BaOH) ionic compound.

Memorizing polyatomic ions? Using Periodic Table Chemistry Stack Exchange
Updated on August 02, 2022 Polyatomic ion definition: A polyatomic ion is an ion composed of two or more atoms. A polyatomic ion has either a positive charge (cation) or negative charge (anion). Examples: The hydroxide cation (OH -) and the phosphate cation (PO 43-) are both polyatomic ions . Cite this Article