
Naming Ionic Compounds Worksheet Pogil —
Naming Ionic Compounds 233. What are the structural units that make up ionic compounds and how are they named? Why? When working in chemistry, it is often convenient to write a chemical in symbols. For example we might write down the substance table salt as NaCl. In talking about chemistry however, it is a bit tacky to say "en-ay see-ell.

12 Naming Molecular Compounds Worksheet Answers /
Terms in this set (3) compound must equal. If a ionic compound forms only one ion then. no change to either name just add ide. If a ionic compound forms multiple ions then. use roman number to specify which ion. Study with Quizlet and memorize flashcards containing terms like compound must equal, If a ionic compound forms only one ion then, If.

Collection Of Naming Molecular Compounds Worksheet Pogil Free
Cations and anions When a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. The result is that the atom becomes a cation —an ion with a net positive charge. For example, the element sodium loses one electron to become a cation:

Naming Ionic Compounds Answer Key Pogil • Suggested and Clear
Naming Ionic Compounds - Nomenclature Rules. When naming ionic compounds, list the cation first and the anion second. The cation is the element name followed by a Roman numeral in parentheses if the element has multiple charges. The anion has the -ide ending for a binary compound or else a polyatomic ion name.

12 Naming Molecular Compounds Worksheet Answers /
Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions.

PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
Naming ionic compounds (practice) | Khan Academy Chemistry library Course: Chemistry library > Unit 1 Lesson 3: Names and formulas of ionic compounds Naming monatomic ions and ionic compounds Common polyatomic ions Polyatomic ions Naming ionic compound with polyvalent ion Worked example: Finding the formula of an ionic compound

Naming Ionic Compounds Worksheet Pogil
Figure 3.10.1 3.10. 1: A Guide to Naming Simple Ionic Compounds. Follow these steps to name a simple ionic compound. Identify the cation name and the anion name. If the cation can have more than one possible charge, either use the Stock system name of the cation and name of the anion, or use the stem of the cation name and -ic/-ous and the name.

Naming Ionic Pounds POGIL Answer Key Polyatomic ion, Writing linear
Table 1. Common polyatomic ions. See page 7 for a table of the ions that you need to know in this class. Cations: + NH4 H3O+ Anions: C2H3O2 - NH2 - N3 - HCO3 - 3- BO3 CO3 2- C2 2- - ClO3 CrO4 2- OCN- CN- Cr2O7 2- Ammonium Hydronium

Naming Acids Chemistry POGIL Answer Key Things to Wear Pinterest
POGIL #1- Ionic Bonds What is an ionic bond? How do they form? Name: Information 1- Electron Dot Structures Electron dot structures- diagrams that show valence electrons as dots. Cations are formed when metals lose their valence electrons to gain an octet, so their electrons aren't shown with dots:

Naming Ionic Compounds Worksheet Pogil
Identify three elements that form only one anion, fechaically vst) N, P, 0, 5, F, C8, LE Identify three elements that form more than one cation, Fe, Ni, Ca, Hg, Sn, Cb d. In what region of the periodic table are these "multiple ion" elements usually located? Center transition Clements (metal) 2. Consider che ions of potassium (K) and sulfur (S).
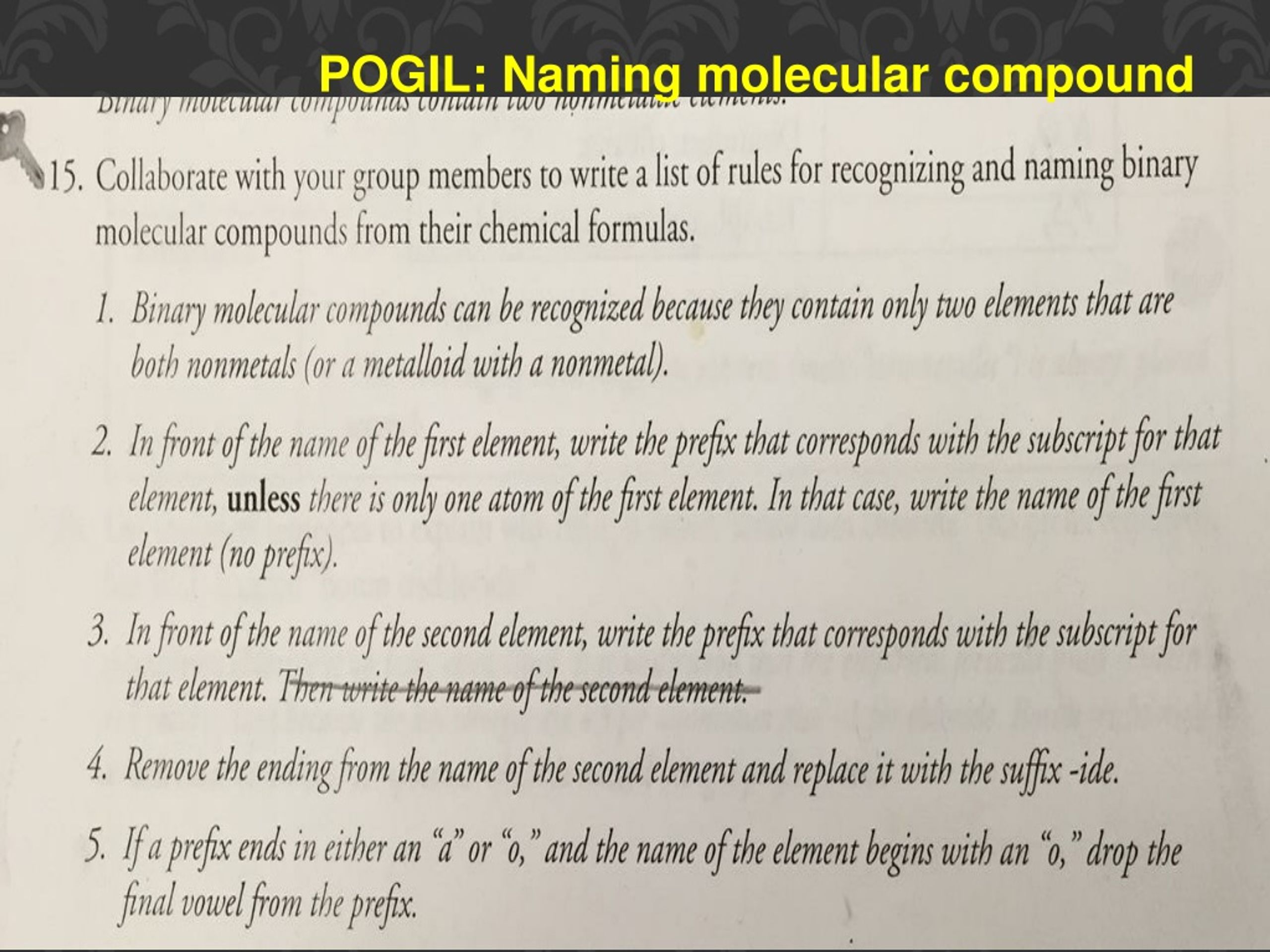
PPT Chemical Bonds Flow Chart POGIL Naming Molecular Compounds
The simplest ionic compounds consist of a single type of cation associated with a single type of anion. Nomenclature for these compounds is trivial; the cation is named first, followed by the anion. If the anion is a single element, the suffix ide is added to the root name of the element. When you are constructing names for ionic compounds, you.

Naming Ionic Compounds Worksheet Pogil
You should be able to write the correct formula for any ionic compound Prerequisites Knowledge of atoms and isotopes Model 1: An atomic look at three compounds The diagrams below represent some ionic compounds at the atomic level. Sodium chloride Chemical formula: NaCl Calcium chloride Chemical formula: CaCl2 POGIL 2005, 2006

Naming Ionic Compounds Worksheet Pogil —
A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion as illustrated in Figure 5.7.1 5.7. 1 for the compound BaCl 2. The word ion is dropped from both parts. Figure 5.7.1 5.7. 1: Naming BaCl2 B a C l 2.

Pogil Naming Binary Ionic Compounds Type I —
Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Image credit: Wikipedia Commons, public domain. Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom.

Naming Ionic Compounds Pogil Extension Questions Answers QUESTIONSF
Ionic Bonding POGIL Goals: 1) Students will use the periodic table to determine the number of electrons in the valance shell of an atom using their knowledge of electron configurations 2) Students will apply the octet rule in determining how various elements combine chemically using the octet rule
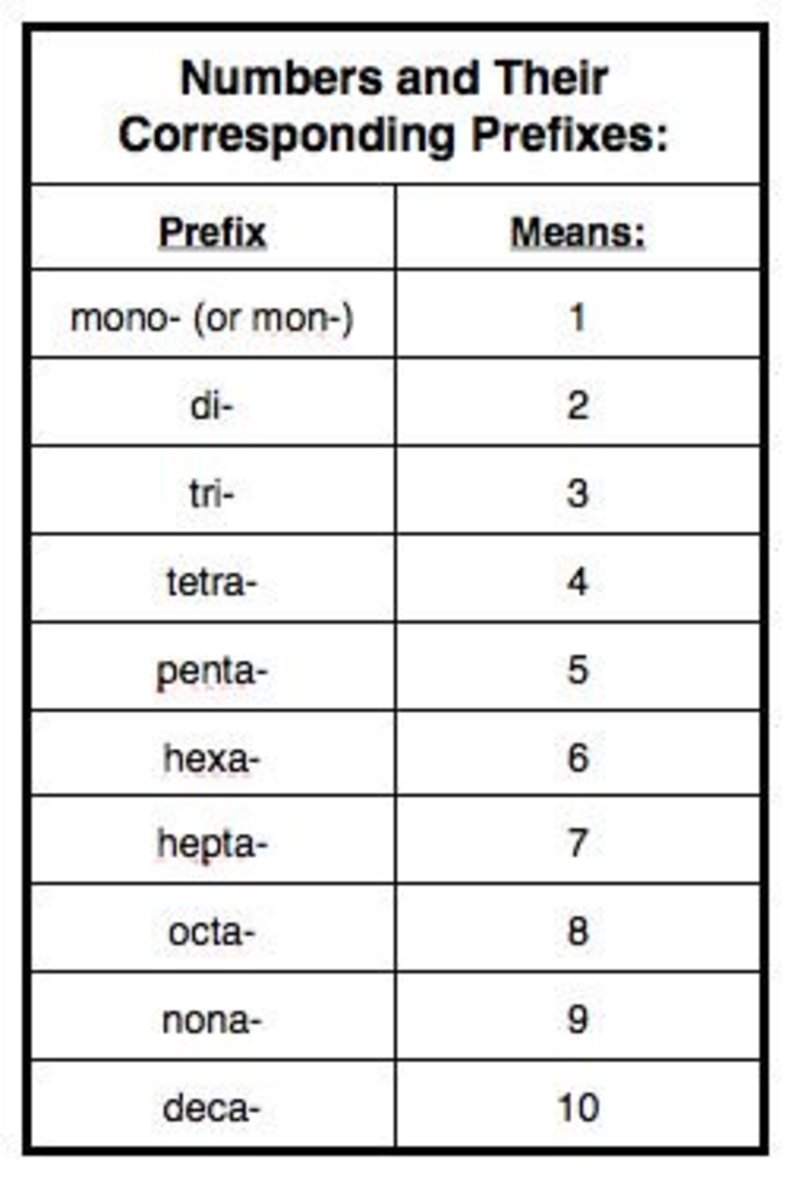
Naming Ionic Compounds Chart
1. Based on the information in Model 1: a. Identify three elements that form only one cation. b. Identify three elements that form only one anion. c. Identify three elements that form more than one cation. d. In what region of the periodic table are these "multiple ion" elements usually located? 2. Consider the ions of potassium (K) and sulfur (S).