
HBr Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
The Lewis structure of HBr contains a single bond between the hydrogen atom and bromine atom. There are three lone pairs on the bromine atom, and the hydrogen atom does not have any lone pair. HBr Lewis Structure - How to Draw the Dot Structure for HBr Watch on Contents Steps #1 Draw skeleton #2 Show chemical bond #3 Mark lone pairs

Is HBr Polar or Nonpolar? (Hydrogen Bromide) in 2021 Molecules, Ball
Share Watch on See the Big List of Lewis Structures Transcript: Hi, this is Dr. B. Let's do the Lewis structure for HBr, hydrobromic acid. On the periodic table, Hydrogen is in group 1, so it has 1 valence electron, and Bromine is in group 7, sometimes called 17, it has 7 valence electrons; for a total of 8 valence electrons.
[Solved] draw a lewis structure for the followings HBr C2HCI Course Hero
A step-by-step explanation of how to draw the HBr Lewis Dot Structure (Hydrogen bromide). For the HBr structure use the periodic table to find the total number of valence electrons.
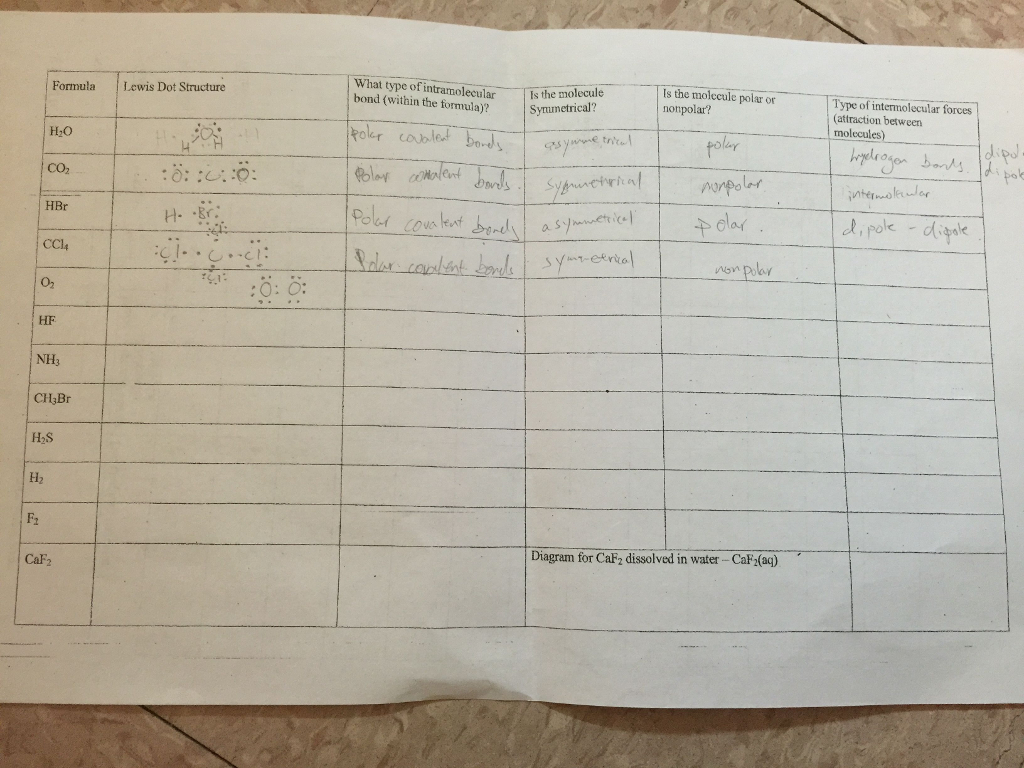
Solved Formula Lewis Dot Structure H2O CO2 HBr H. ..Br C...
Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond.
[Solved] draw a lewis structure for the followings HBr C2HCI Course Hero
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide (HBr). It showcases the connectivity between hydrogen (H) and bromine (Br) atoms, giving us a visual representation of their covalent bond. Atomic Information: Hydrogen (H): 1 proton, 1 electron Bromine (Br): 35 protons, 35 electrons
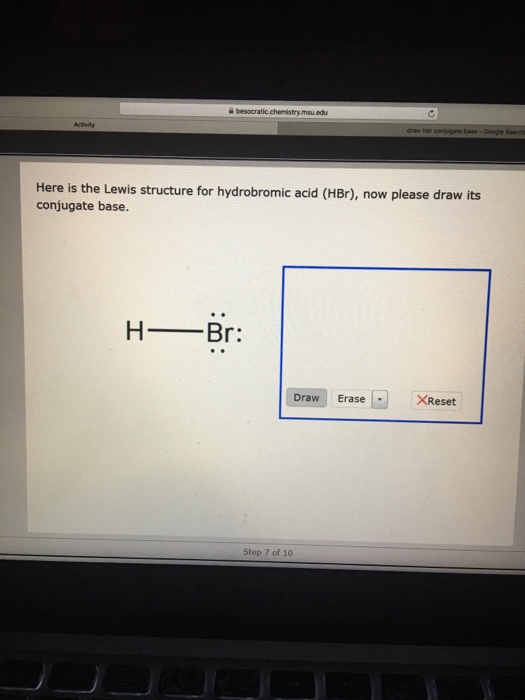
Solved Here is the Lewis structure for hydrobromic acid
1.Determine the number of lone pairs of electrons in the core bromine atom of the HBr Lewis structure. Because the lone pairs of electrons on the bromine atom are mostly responsible for the HBr molecule geometry planar, we need to calculate out how many there are on the central bromine atom of the HBr Lewis structure.

Best Overview Is HBr Polar or Nonpolar? Science Education and Tutorials
Exercise 10.4.1 10.4. 1. Use Lewis electron dot diagrams to illustrate the covalent bond formation in Cl 2. Answer. When working with covalent structures, it sometimes looks like you have leftover electrons. You apply the rules you learned so far, and there are still some electrons that remain unattached.

Draw the Lewis Structure of HBr (hydrogen bromide) YouTube
What is the lewis dot structure for hydrogen bromide (HBr)? The total number of electrons would 8. There is a single bond connecting hydrogen and bromine. Since hydrogen is satisfied by only two electrons, the rest of the lone pairs will end up on bromine. What is the lewis dot structure of carbon tetrachloride (CCl 4)? There are 32 valence.

Hbr Lewis Structure Transborder Media
Write Lewis structures for the following:(a) H2(b) HBr(c) PCl3(d) SF2(e) H2CCH2(f) HNNH(g) H2CNH(h) NO-(i) N2(j) CO(k) CN-OpenStax™ is a registered trademark.
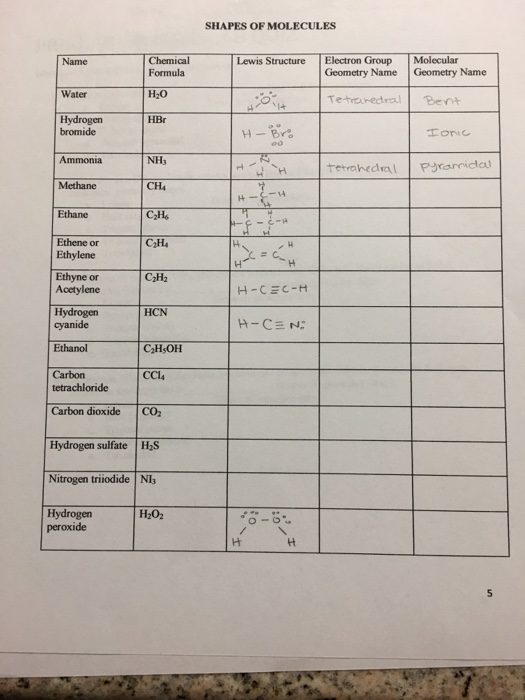
Solved SHAPES OF MOLECULES Chemical Lewis Structure Electron
Connect each atom to the central atom with a single bond (one electron pair). Subtract the number of bonding electrons from the total. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom. Place all remaining electrons on the central atom.

Hbr Lewis Structure Transborder Media
Steps of drawing HBr lewis structure Step 1: Find the total valence electrons in HBr molecule. In order to find the total valence electrons in HBr (hydrogen bromide) molecule, first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Is HBr Polar or Nonpolar? (Hydrogen bromide) YouTube
Step 1 To find out the Lewis Structure of any given molecule, the first step is to find out the total valence electron number. Electron loss signifies the increase of positive charge hence we use the sign '+'. The gain of electrons increases the number of negatively charged electrons therefore we use the '-' sign. Step 2

Lewis Structure Hbr
Lewis structure of Hydrogen bromide (HBr) contains only one H-Br bond. There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. HBr lewis structure

Hydrogen bromide (HBr) molecule. Skeletal formula Stock Vector Image
The first step is to sketch the Lewis structure of the HBr molecule, to add valence electrons around the bromine atom; the second step is to add valence electrons to the one hydrogen atom, and the final step is to combine the step1 and step2 to get the HBr Lewis Structure.

Lewis Dot Structure For Hbr
Lewis structure of HBrO contains a single bond between the Hydrogen (H) & Oxygen (O) atom as well as between the Oxygen (O) and Bromine (Br) atom. The Oxygen atom (O) is at the center and it is surrounded by Hydrogen and Bromine atom. The Oxygen has 2 lone pairs and the Bromine has 3 lone pairs.

Hbr Lewis Structure Transborder Media
In the HBr Lewis structure, there is a single bond between the hydrogen and bromine atom, and on the bromine atom, there are three lone pairs. HBr Lewis Structure - How to Draw the Dot Structure for HBr Watch on Contents Steps #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms