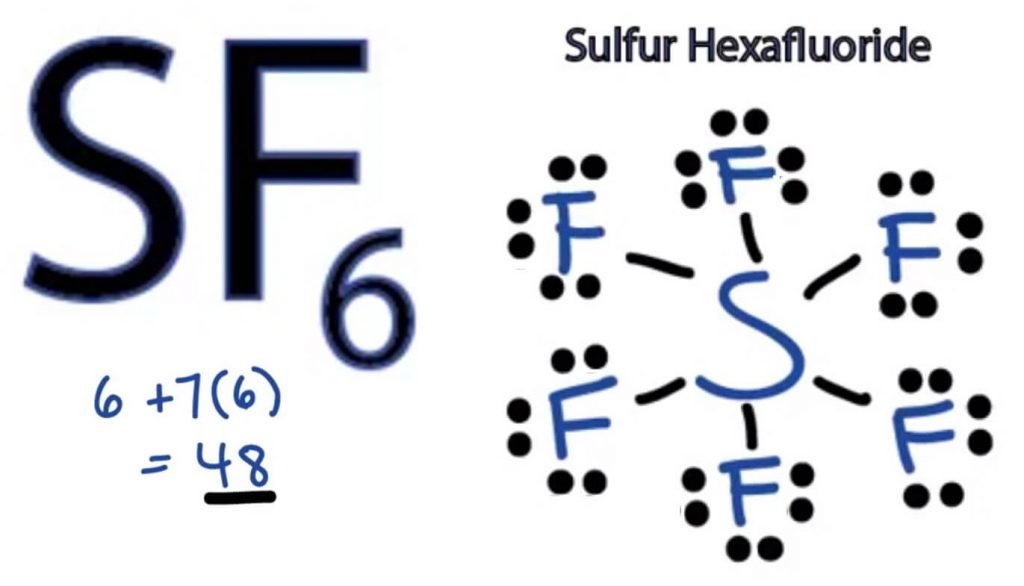
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity
Contents SF6 Valence Electrons SF6 Lewis Structure SF6 Hybridization SF6 Bond angle SF6 Molecular Geometry SF6 Shape Is SF6 polar or nonpolar? SF6 Valence Electrons To determine the Lewis Structure of any molecule, we first need to know the total number of valence electrons.

MakeTheBrainHappy Is SF6 Ionic or Covalent?
So, the final answer is: $\textbf{The molecule $\mathrm{SF}_{6}$ is nonpolar due to the symmetrical arrangement of polar bonds which cancel out each other's dipole moments. However, $\mathrm{SF}_{5} \mathrm{Br}$ is polar because the presence of a non-equivalent bond results in a net dipole moment for the molecule.}$

Sf6 Polar Atau Nonpolar Brain
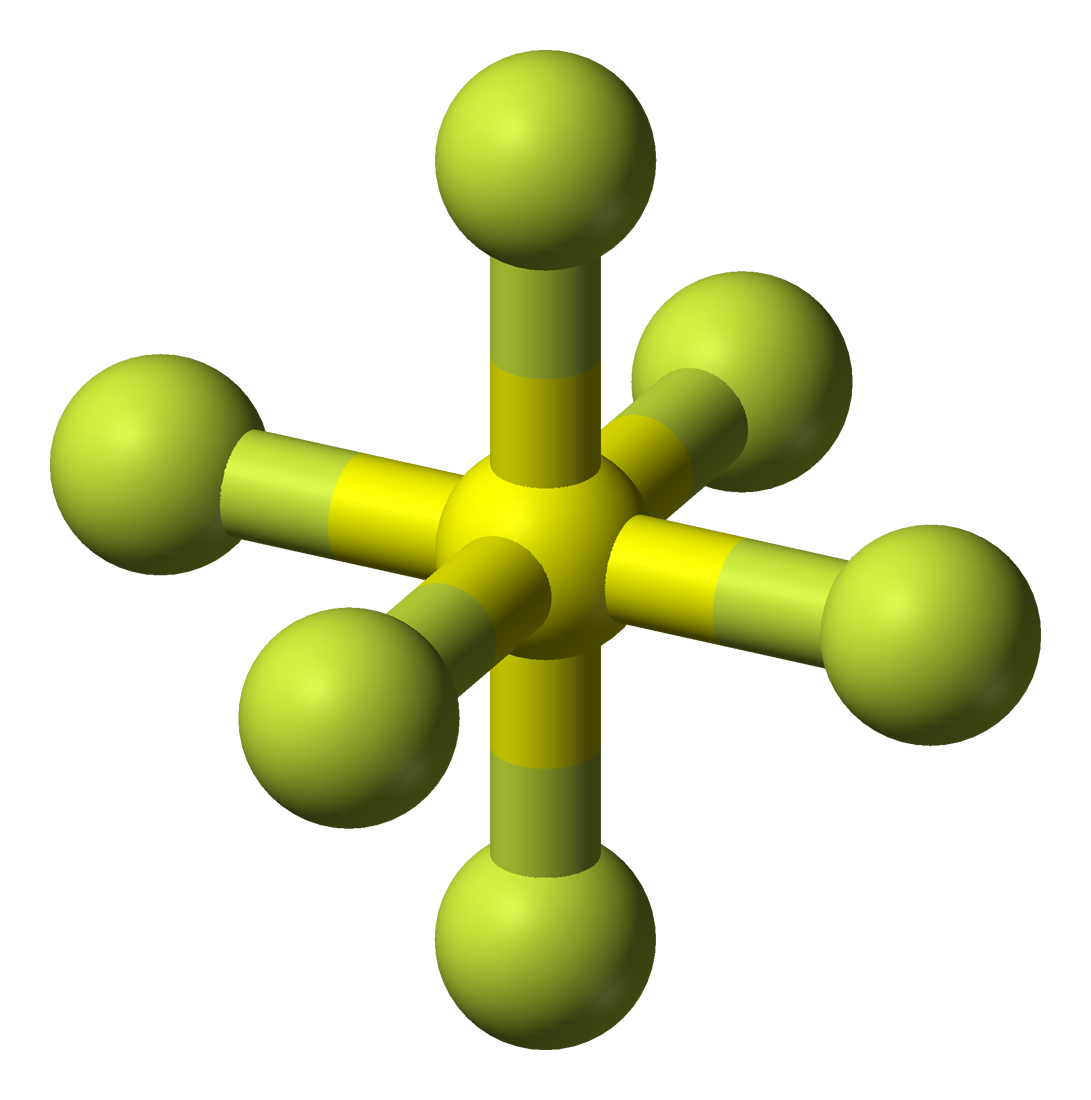
SF6 is a NONPOLAR molecule because all the six bonds (S-F bonds) are identical and SF6 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SF6 lewis structure and its 3D geometry. Why is SF6 a Nonpolar molecule? (Explained in 3 Steps)

Sf6 Polar Atau Nonpolar Brain
SF6 is a nonpolar compound in nature because as per VSEPR theory six fluorine atoms are arranged symmetrically with the sulfur atom such that dipole moment of S-F bond gets canceled out making the SF6 a nonpolar compound. SF6 has its IUPAC name Sulfur hexafluoride and it is considered as an extreme gas responsible for the greenhouse effect.
Sf6 Polar or Nonpolar Jessica McLean
Draw Lewis dot (electron) structure for SF6 and determine the following: a) electron geometry b) molecular geometry c) central atom hybridization d) polar or nonpolar This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.

Sf6 Polar Or Nonpolar Polar and Nonpolar Molecules Is it Polar or Nonpolar What are
This answer is: More answers Wiki User ∙ 14y ago Copy SF6 is non-polar. Reason: Fluorine is more electronegative than sulfur, so the bond dipoles point toward fluorine. However, all the six S-F.

MakeTheBrainHappy Is SF6 Polar or Nonpolar?
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.

Sf6 Polar Atau Nonpolar Brain
Sulfur hexafluoride, abbreviated as SF6, is a nonpolar molecule. SF6 has an octahedral molecular geometry, which means that the sulfur molecule has six fluorine atoms surrounding it. While each individual bond is polar, there is no net effect, meaning that the molecule is nonpolar.

Sf6 Polar Atau Nonpolar Brain
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Is SF6 polar or nonpolar? YouTube
SF6 Lewis Structure has six single bonds surrounding the Sulphur atom, which has six fluorine atoms linked to it and three lone pairs on each fluorine atom while there is no lone pair on the Sulphur atom.. As a result, sulfur hexafluoride is non-polar. Factors affecting polarity. Electronegativity; The stronger the atom's ability to.

Sf6 Polar Atau Nonpolar Brain
Is SF6 an ionic, polar, or nonpolar compound? Click the card to flip 👆 Nonpolar Click the card to flip 👆 1 / 36 Flashcards Learn Test Match Q-Chat Created by aaesoph Teacher Students also viewed Electronegativity and Polarity 30 terms jksandhu Preview Gen Chem 1 - Covalent Bonding 16 terms arduat Preview CHEM 55 terms silentsilhouette Preview

Sf6 Polar Atau Nonpolar Brain
SF6 (Sulfur hexafluoride) is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, S is a nonmetal and F is also a nonmetal. So when they combine, it forms a covalent compound.

Sf6 Polar Atau Nonpolar Brain
Learn to determine if SF6 (Sulfur hexachloride) is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.

Is SF6 Polar or Nonpolar? Techiescientist
SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. The general identification of the gas can't be done because it is odorless and colorless in nature. There is also no taste of the gas as such. SF6 is noncombustible and nonflammable in nature.
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity
Is S F 6 a polar or nonpolar molecule? Explain. Question: Is S F 6 a polar or nonpolar molecule? Explain. Polar Molecule: Smaller covalent molecules often only contain a single central.

Is SF6 (Sulfur hexafluoride) Polar or NonPolar? YouTube
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.