
Phase Diagrams YouTube
A binary phase diagram shows the phases formed in differing mixtures of two elements over a range of temperatures. Compositions run from 100% Element A on the left of the diagram, through all possible mixtures, to 100% Element B on the right. The composition of an alloy is given in the form A - x%B.
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Resource Book
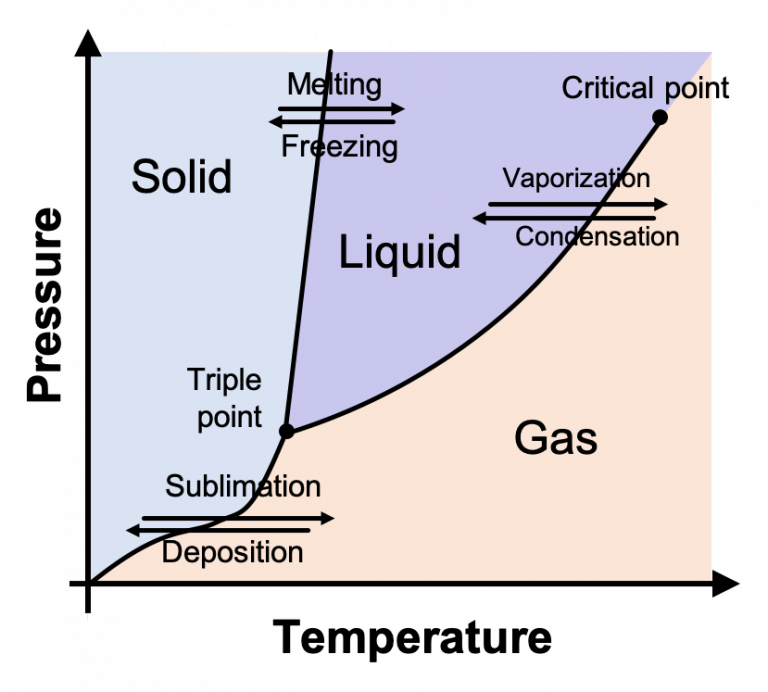
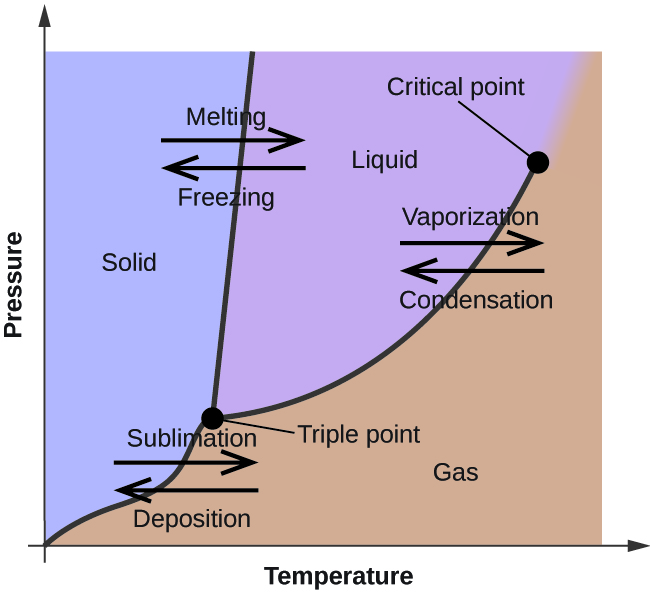
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid.
Phase Diagrams Chemistry
While this may seem like an obscure question, the MCAT specifically requires you to know the shape of the water phase diagram. Unique to only a few molecules, the solid phase in our diagram is less dense than the liquid phase, meaning that the solid/liquid phase line has a negative slope (this can be seen as segment AD in the above image).

Phase Diagram of CO2 Change Printable Diagram Diagram, Co2, Printables
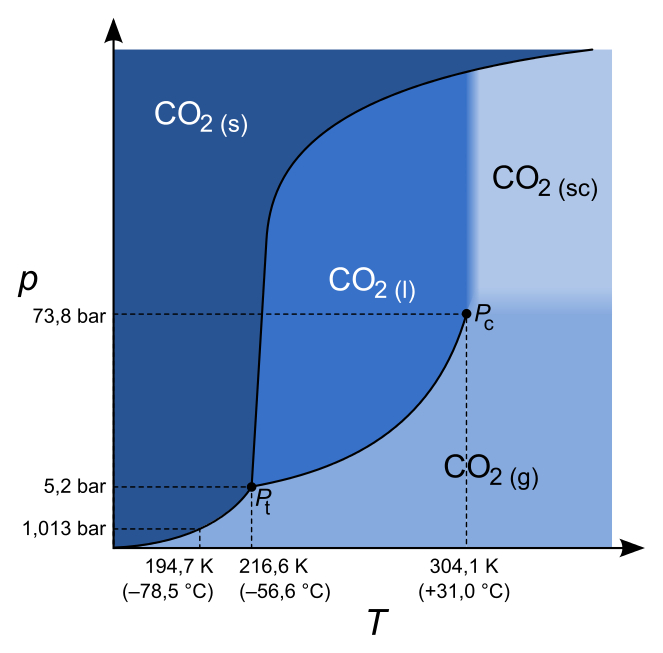
Draw the phase diagram using the following data: (a) triple point = 10°C, 0.05 atm (b) critical point = 225°C, 15 atm (c) normal melting point = 50°C (d) normal boiling point = 120°C (e) Sublimation at 0°C requires 0.005 atm. Identify the correct statement below: I. Deposition is represented by line AB. II.
.PNG)
Phase Diagrams Presentation Chemistry
4.4 Multi-Component Phase Diagrams. So far we have only dealt with phase diagrams of pure components but typically you will deal with either binary, ternary, quaternary, etc. phase diagrams.. Let's take a look at a relatively simple phase diagram, a Binary Lens phase diagram which holds for ideal solution scenarios. (Side Note: When I say something is simple please do not interpret this as.

How To Draw A Phase Diagram Images and Photos finder
[1] is the temperature below which the substance is stable in the solid state. The is the temperature above which the substance is stable in a liquid state. There may be a gap between the solidus and liquidus; within the gap, the substance consists of a mixture of crystals and liquid (like a " [2]

Engineering Materials_Chapter6_Phase Diagram _4 YouTube
A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance. This is the phase diagram for a typical pure substance. These diagrams (including this one) are nearly.

phase Thermodynamics of SolidLiquid Chemistry Stack Exchange
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/states-of-matter-a.
How do you draw a phase diagram with a differential equation? Socratic
phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions "equilibrium" is important: phase diagrams are determined by using slow cooling conditions ⇒ no information about kinetics

Matlab Creating a Ternary Phase Diagram Using MATLAB
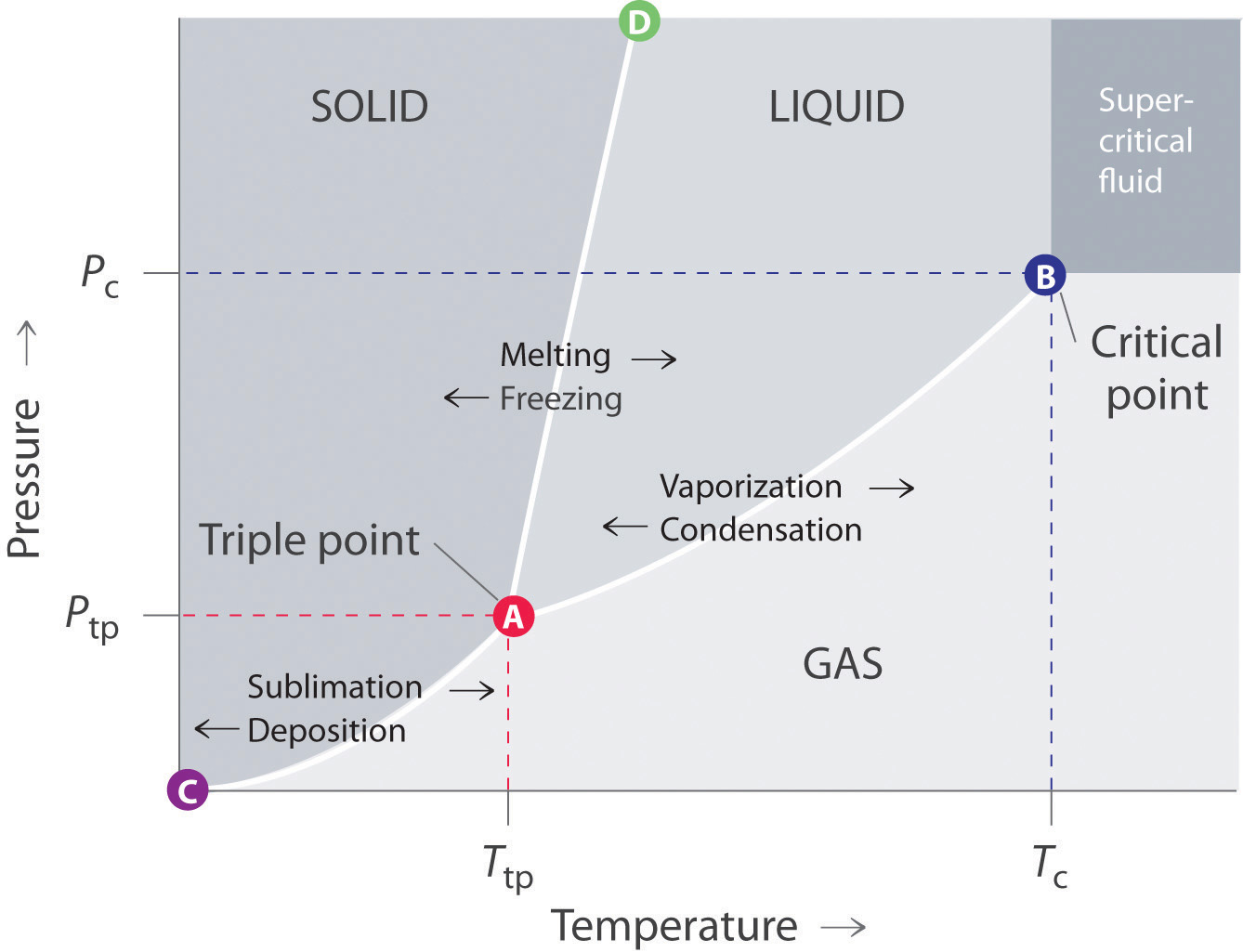
A typical phase diagram for a pure substance is shown in [link]. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in [link].
How do you draw a phase diagram with a differential equation? Socratic
0:00 / 6:36 Phase diagrams are a graph that relates the pressure and temperature of a substance to the state of matter (solid, liquid or gas).Thanks for watching. Please.
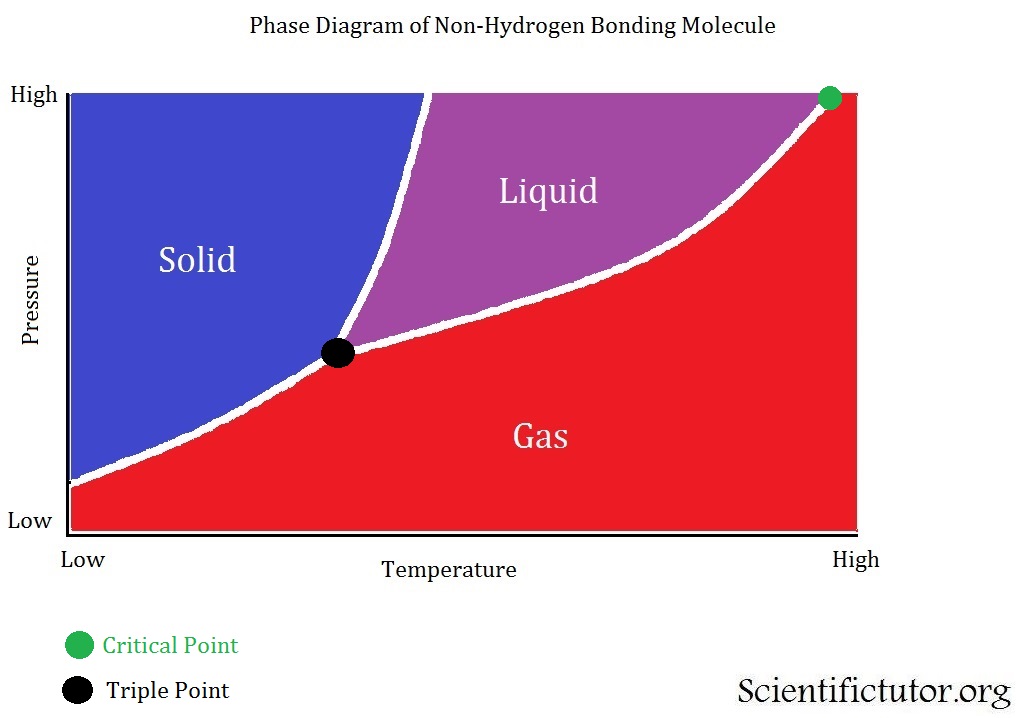
Chem Phase Diagrams Scientific Tutor
Example 12.4. 1: Water. Referring to the phase diagram of water in Figure 12.4. 2: predict the physical form of a sample of water at 400°C and 150 atm. describe the changes that occur as the sample in part (a) is slowly allowed to cool to −50°C at a constant pressure of 150 atm.
Phase Diagrams CK12 Foundation
Lesson 1: States of matter States of matter States of matter follow-up Specific heat and latent heat of fusion and vaporization Specific heat, heat of fusion and vaporization example Chilling water problem Change of state example Vapor pressure Phase diagrams Representing solids, liquids, and gases using particulate models
:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)
Phase Diagrams Phases of Matter and Phase Transitions
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist.
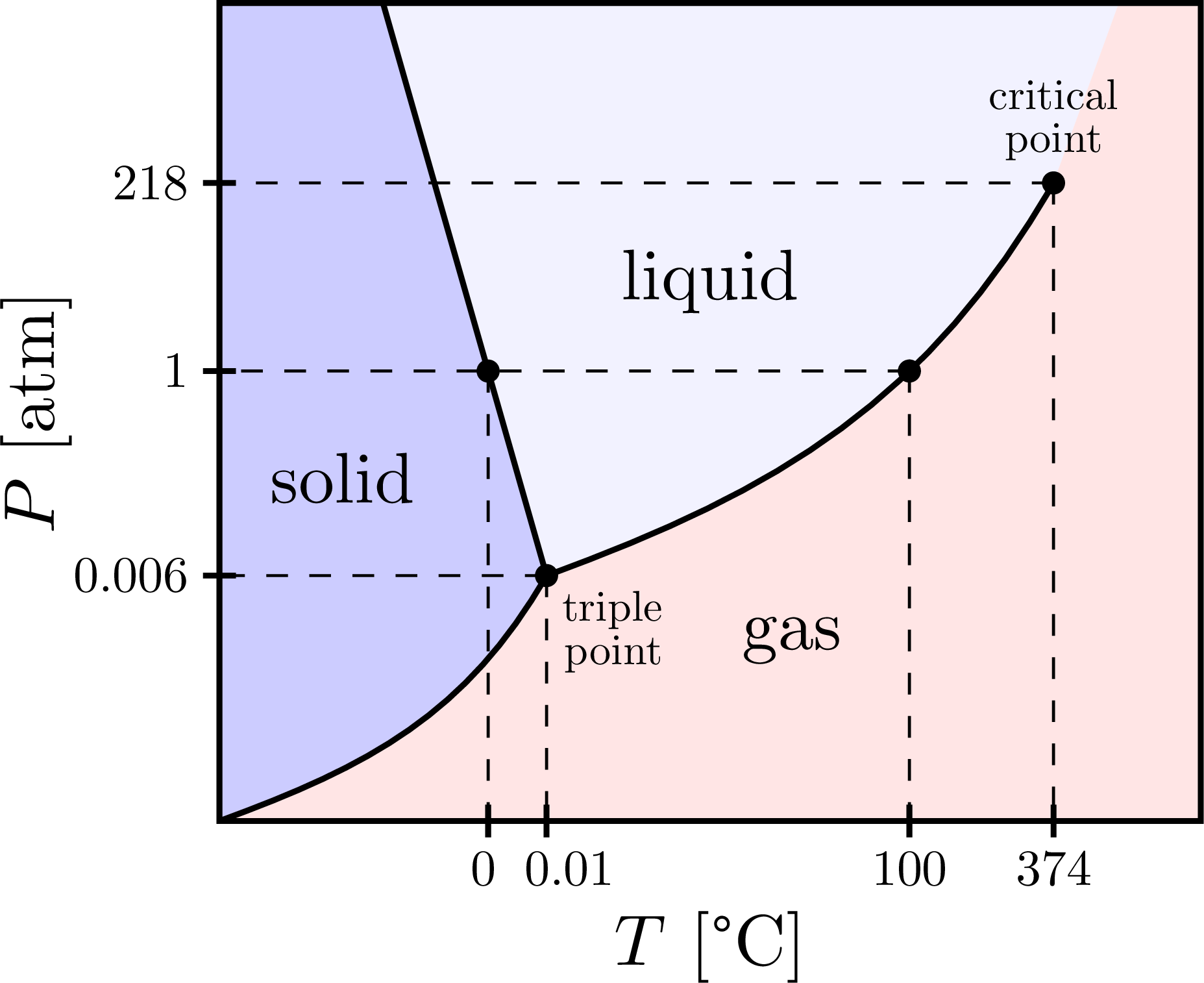
Phase transitions
A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.

Phase Diagram Definition Of A Phase Diagram Images
draw a phase diagram Natural Language Math Input Extended Keyboard Examples Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music…