
PPT AS Chemistry PowerPoint Presentation, free download ID2093824
Molecules having their charges unevenly distributed are said to be polar in nature, whereas, those molecules whose charges are evenly distributed are non-polar. In case of CCl4, the charges are distributed uniformly around the central carbon atom. Due to this symmetry, tetrachloromethane is said to be non-polar, even though it contains polar bonds.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry quotes, Covalent bonding
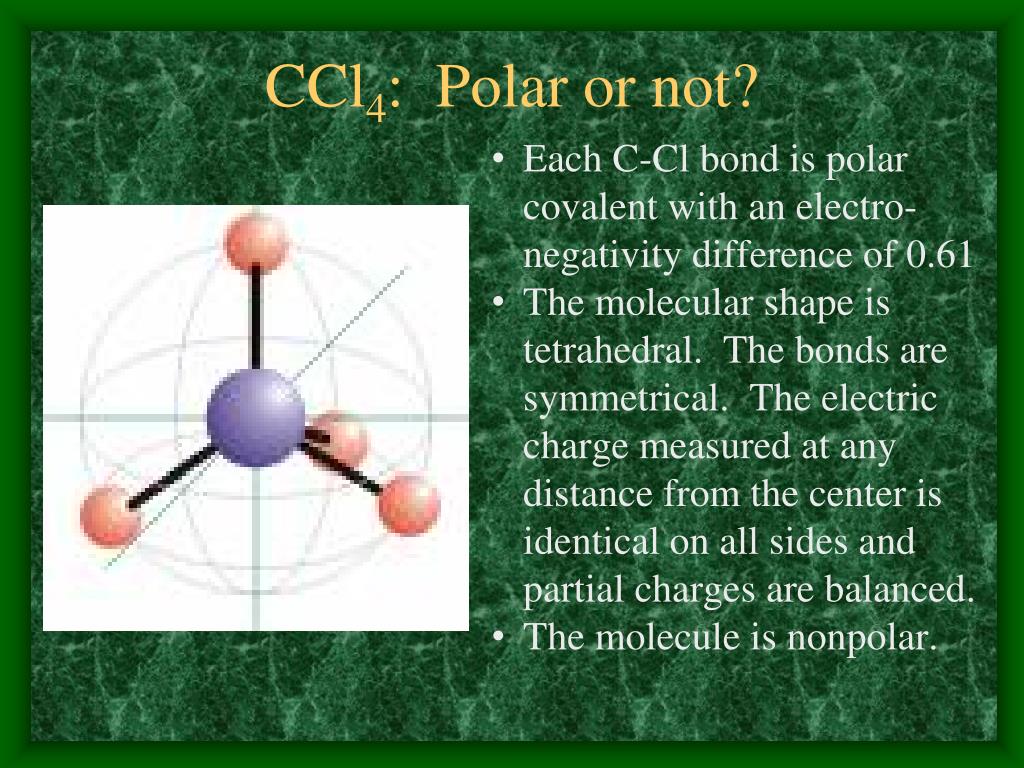
Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl 4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane.

Is CCl4 Polar Or Nonpolar?
Answer: CCl4, a carbon tetrachloride, is stated to be nonpolar. The reason for this is that all four bonds in carbon tetrachloride are symmetrical and also, and they are extended in all directions. It is due to this same fact that it becomes easy for the dipole moment of one bond to cancel out the other placed in the opposite direction.

Polar and Nonpolar Molecules
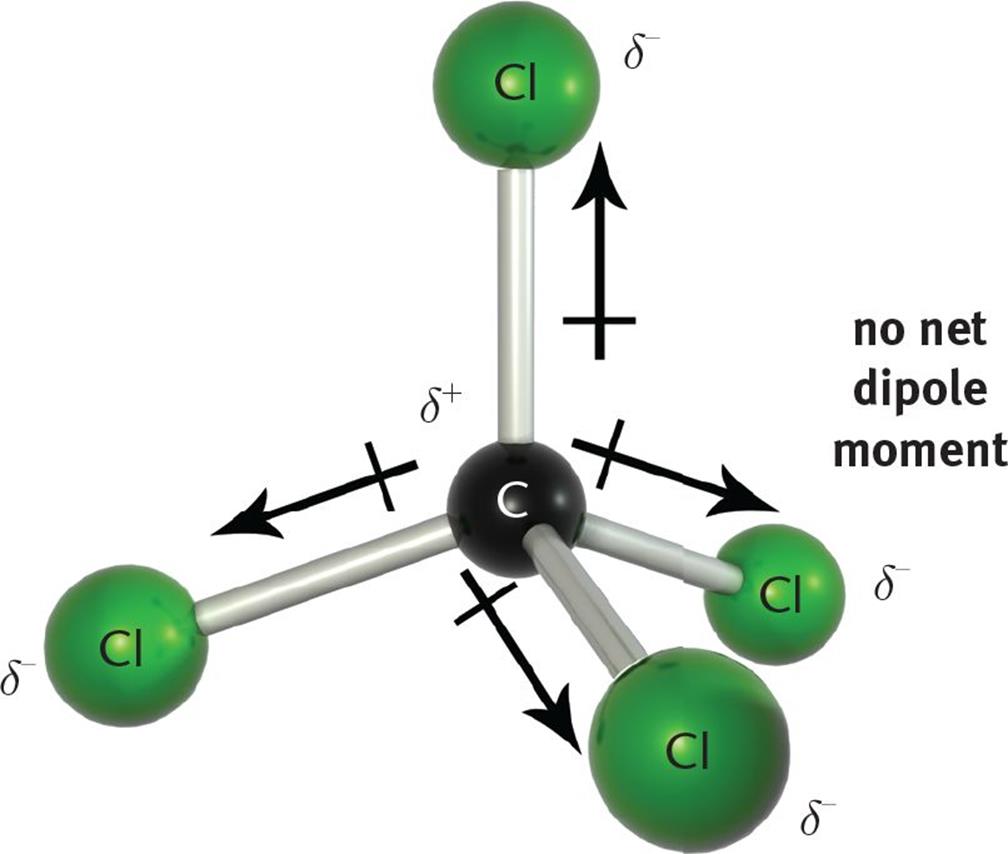
So for CCl4, the electronegativity difference (ΔEN) = 3.16 - 2.55 = 0.61. This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Chlorine (Cl) is polar covalent bond. But if you look at the 3D structure of CCl4, you can see that the structure of CCl4 is symmetrical. As both the bonds (C-Cl) are symmetrical.
MakeTheBrainHappy Is CCl4 Polar or Nonpolar?
Carbon tetrachloride (CCl4) is a non-polar molecule. There are four C-Cl polar bonds present in CCl4. The polarity of each bond is attributed to a significant electronegativity difference between the two bonded atoms. The whole molecule however is non-polar due to its symmetric, tetrahedral shape.

bcl3 polar or nonpolar News City Fit
When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. The degree to which.
Difference between polar and nonpolar examples
Carbon tetrachloride is a polar molecule because it has a permanent dipole. This means that one side of the molecule is positively charged and the other side is negatively charged. These charges are intensified by the electron cloud distortion induced by the strong carbon-chlorine bond. Is CCl4 Polar or Non-Polar? (Explained)

Cara Membedakan Polar Dan Nonpolar
Polar or Nonpolar Nature of CCl4: CCl4 is a nonpolar molecule. While it contains polar C-Cl bonds due to the difference in electronegativity between carbon and chlorine, the symmetric tetrahedral molecular shape results in the cancellation of dipole moments, leading to a nonpolar molecule overall. Hybridization in CCl4:
Simple Question Do all polar molecules have a dipole moment? r/chemhelp
Is CCl4 Polar or Non-polar? (Carbon Tetrachloride) Wayne Breslyn 724K subscribers Join Subscribe Subscribed 537 66K views 5 years ago Learn to determine if CCl4 is polar or nonpolar.
PPT Covalent Bonding PowerPoint Presentation, free download ID5759515
Is CCl4 polar or nonpolar? Question: Is CCl 4 polar or nonpolar? Polar Molecules: Polar molecules are molecules which have an uneven distribution of charge. They are often produced as a.

Best Overview Is HCl polar or nonpolar [1] Science Education and Tutorials
Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct.

Hat Is the Name of the Covalent Compound Ccl4
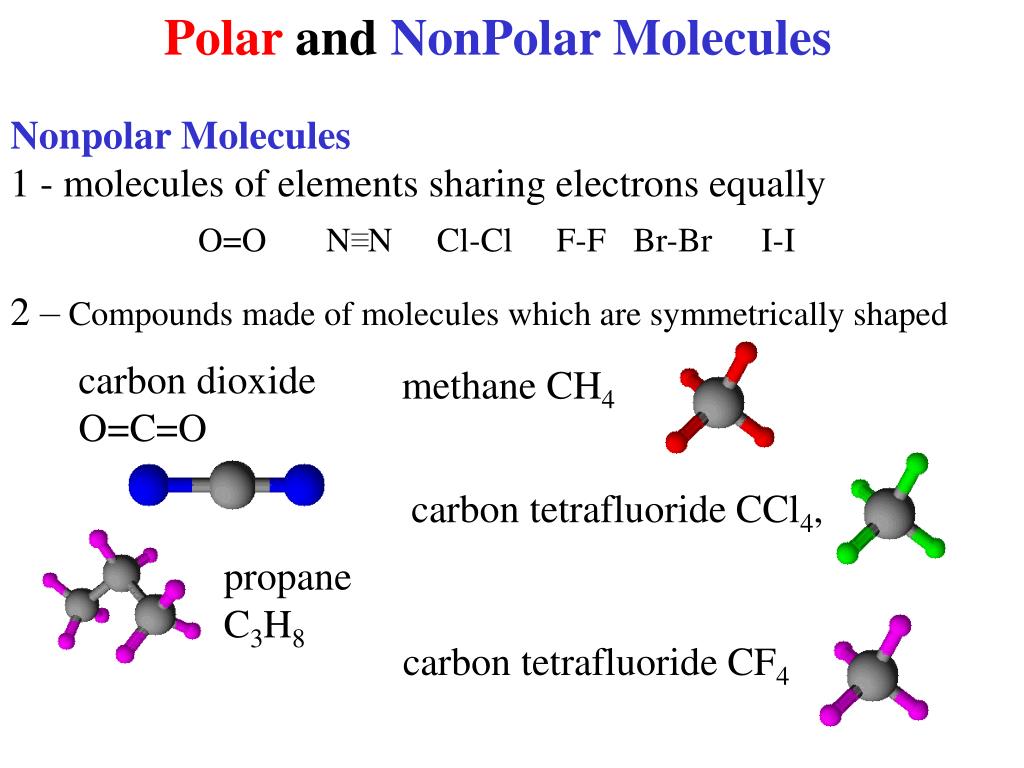
Notice that a tetrahedral molecule such as CCl4 CCl 4 is nonpolar Figure ( 4.12.1 4.12. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ).

Is CCl4 Polar Or Nonpolar?
Is CCl 4 Polar Or Nonpolar? Solution Carbon tetrachloride (CCl4) is an organic compound that can be formed by the halogenation reaction between methane and chlorine in the presence of sunlight. In this compound, the chlorine is arranged in a tetrahedron from around the carbon atom, giving a symmetric arrangement.
PPT Polar and NonPolar Molecules PowerPoint Presentation, free download ID6695002
Polar or Nonpolar Carbon tetrachloride (CCl4) is a chemical compound that consists of one carbon atom bonded to four chlorine atoms. When determining the polarity of a molecule, we consider the molecular geometry and the electronegativity difference between the atoms.
21. Which among CH4 and CCl4 is highly polar and why
CCl4 (or Carbon tetrachloride) is a NONPOLAR molecule because all the four bonds (C-Cl bonds) are identical and CCl4 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of CCl4 lewis structure and its 3D geometry. Why is CCl4 a Nonpolar molecule? (Explained in 3 Steps)

Ccl4 Polar or Nonpolar EmersonminJacobs
Hey Guys!In this video, we are going to determine the polarity of Carbon Tetrachloride having a chemical formula of CCl4. It is made of one Carbon atom and f.