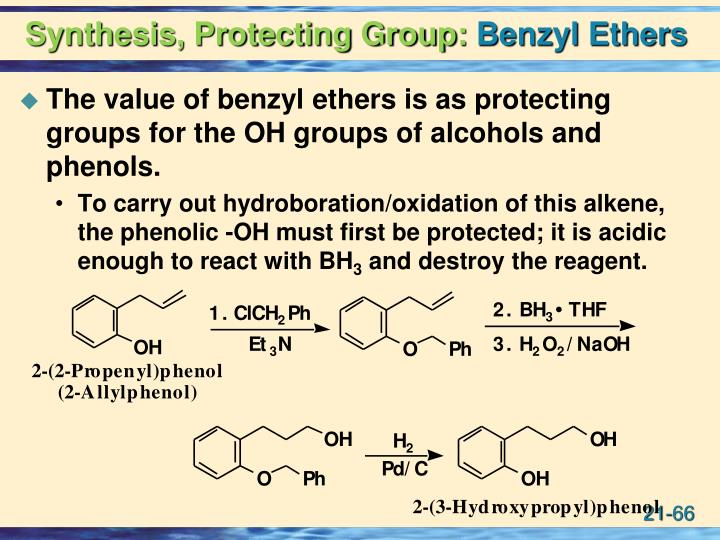
Benzyloxy carbamate (CBz)protecting group.
Common protecting groups Alcohol protecting groups Protection of alcohols : Protection of alcohol as tetrahydropyranyl ether followed by deprotection. Both steps require acid catalysts. Acetyl (Ac) - Removed by acid or base (see Acetoxy group ). Benzoyl (Bz) - Removed by acid or base, more stable than Ac group.

(a) Cys thiol protection with the benzyl (Bn/Bzl) protecting group (b)... Download Scientific
A comparison of benzyl and 2-naphthylmethyl ethers as permanent hydroxyl protecting groups in the synthesis of α-galactoglycosphingolipids KRN7000 and PBS-57. Journal of Carbohydrate Chemistry 2017 , 36 (4-6) , 173-188.
Acid Disodium Pyrophosphate Benzyl Group Protecting Group, PNG, 720x600px, Acid, Amino Acid
Remote Electronic Effects by Ether Protecting Groups Fine-Tune Glycosyl Donor Reactivity. The Journal of Organic Chemistry 2016, 81 (12). 1,2- cis -Selective glucosylation enabled by halogenated benzyl protecting groups. Organic & Biomolecular.
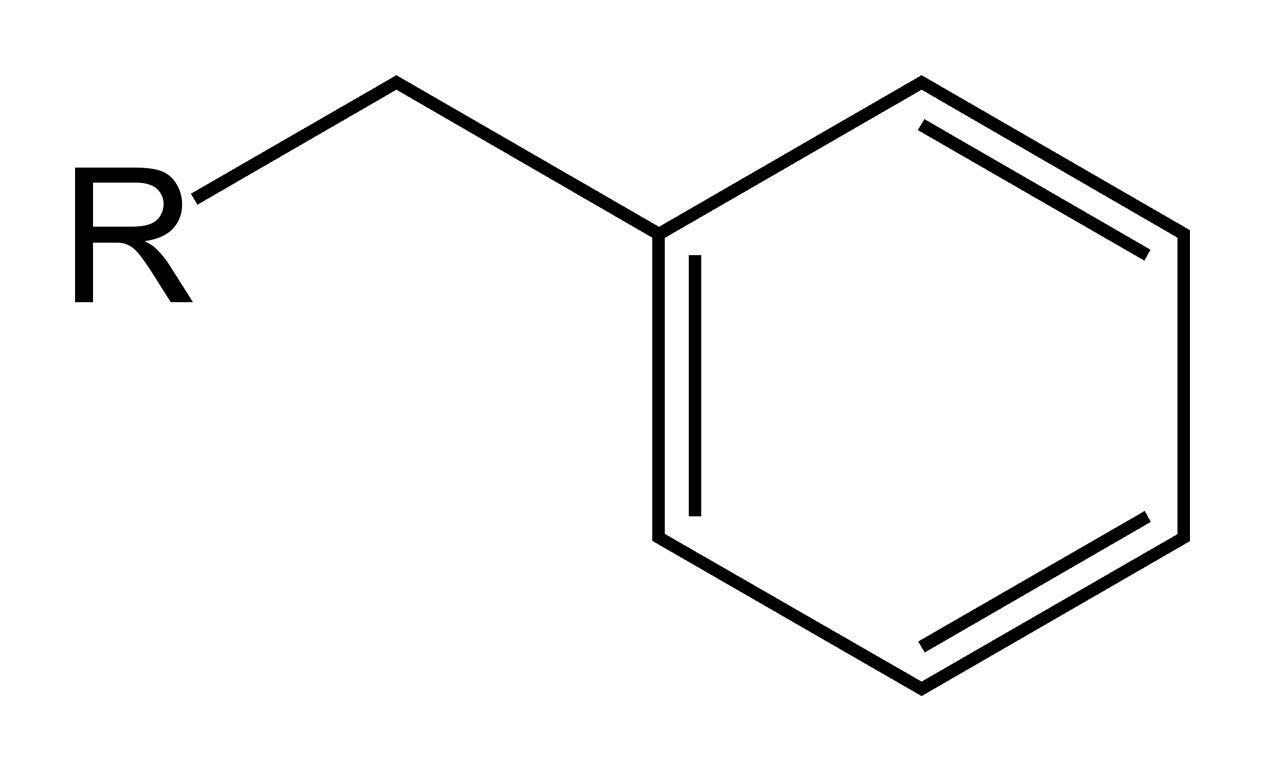
Benzyl ether (Bn)protecting group.
The protecting group on a carbohydrate plays the pivotal role in modulating the reactivity of the (mono)saccharide, and this section will describe how protecting group can be used to control stereoselective transformations (most importantly, glycosylation reactions) and reactivity-driven one-pot synthetic strategies.
PPT Aromatic Nomenclature PowerPoint Presentation, free download ID5172827
Functional Groups: Amino Carbonyl Carboxyl Hydroxyl ( 1,2-; 1,3-Diols) What are protective groups? A protective group (also referred to as "protecting group") is a reversably formed derivative of an existing functional group in a molecule.
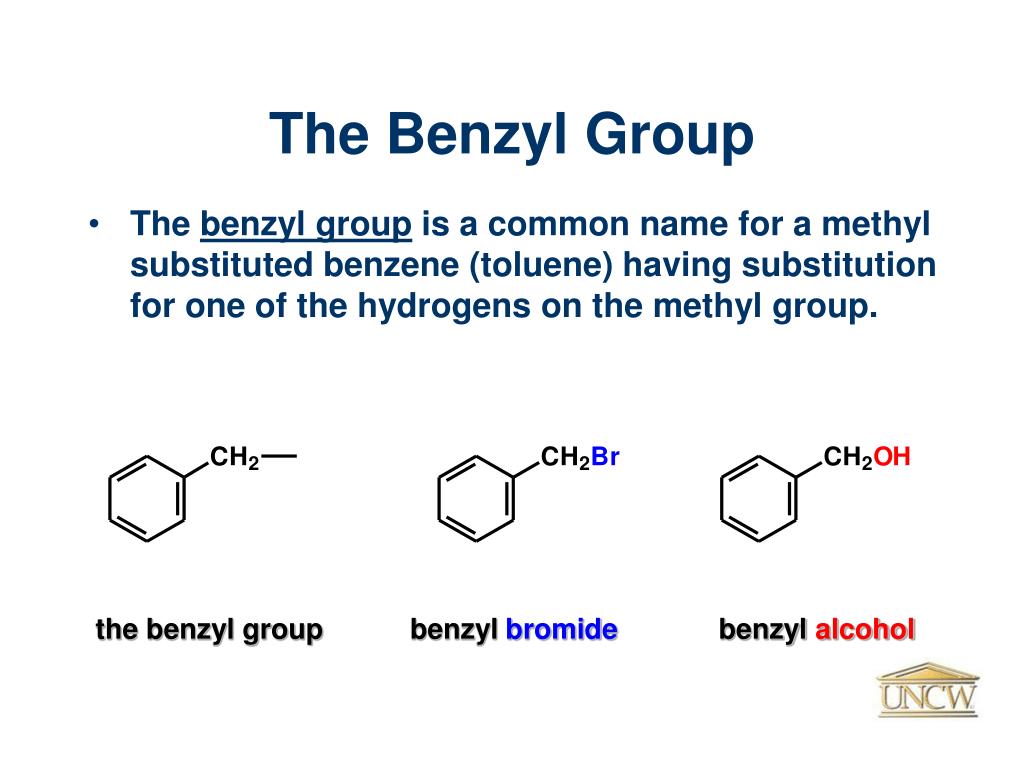
benzyl group Liberal Dictionary
Other Syntheses of Benzyl-Protected Amino Groups A highly efficient general strategy for the synthesis of 2-amino acids by homologation of α-amino acids, involving the Reformatsky reaction with a Mannich-type imminium electrophile is reported. R. Moumne, S. Lavielle, P. Karoyan, J. Org. Chem., 2006 , 71, 3332-3334. Deprotection

reactive SN2 alkyl groups benzyl and allyl groups YouTube
The benzyl group as well as its derivatives are widely adopted as protecting groups in chemical synthesis. Most of the debenzylation protocols are realized by transition-metal catalyzed hydrogenolysis or Birch reduction. However, the flammability of hydrogen and alkalis, harsh conditions, and low functional-group compatibility impede its utility.
Benzyl ether (Bn)protecting group.
75 of The Top 100 Retailers Can Be Found on eBay. Find Great Deals from the Top Retailers. eBay Is Here For You with Money Back Guarantee and Easy Return. Get Your Benzyl Today!

Benzyl ether (Bn)protecting group.
A list of typical conditions for benzyl deprotection. 1) Kocienski, P. J.; Protecting Groups, 3rd Edition 2) Wuts, P. G. M.; Greene, T. W.; Greene's Protective Groups.
BSTFA Protecting Group Chemical Compound Benzyl Group Chemical Formula, PNG, 1200x1017px
1.2 Requirements for Protecting Groups The use of a protecting group adds two steps to a synthesis: One for protection, the other one for deprotection. Both steps need to be virtually quantitative to not significantly affect the overall yield of the synthesis.
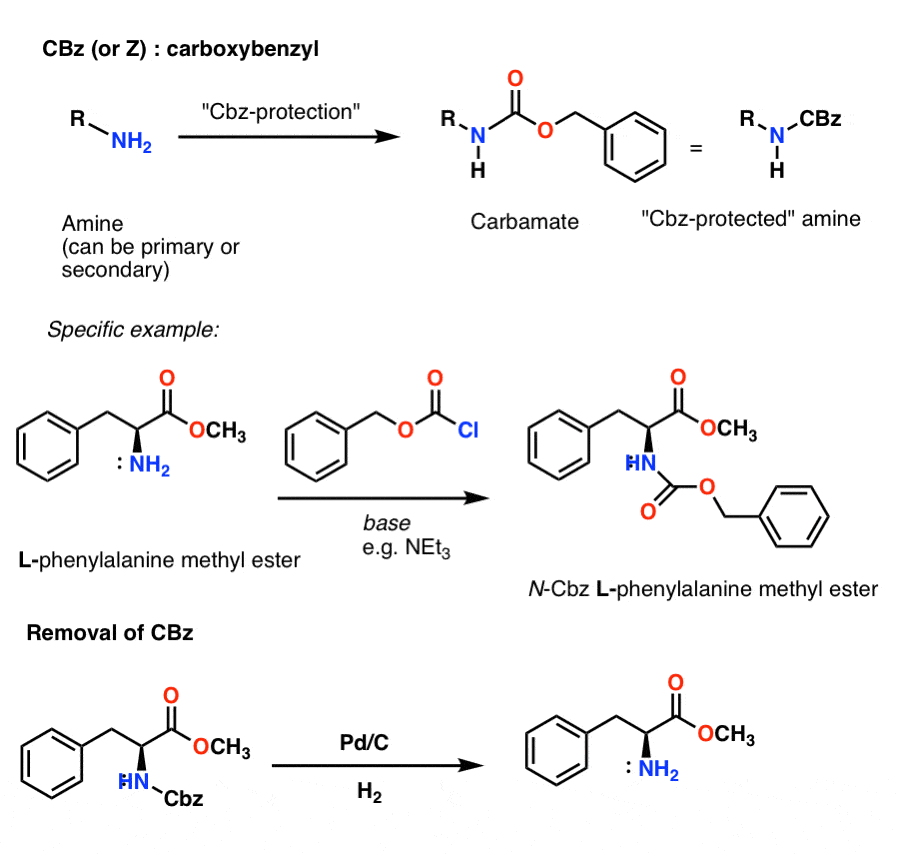
Protecting Groups for Amines Carbamates Master Organic Chemistry
Some Common Protecting Groups in Organic Synthesis. Hydroxyl (OH) ( OH) protecting groups in Organic Synthesis. Protection of alcohols: Acetyl (Ac) ( Ac) - Removed by acid or base. Benzoyl (Bz) ( Bz) - Removed by acid or base, more stable than Ac Ac group. Benzyl ( Bn Bn, Bnl Bnl) - Removed by hydrogenolysis. Bn Bn group is widely used in.
PPT Chapter 21, Benzene and and the Concept of Aromaticity PowerPoint Presentation ID142374
H. Sajiki, Tetrahedron Lett., 1995 , 36, 3465-3468. Benzyl esters of various acids can be chemoselectively cleaved on treatment with nickel boride in methanol at ambient temperature to give the parent carboxylic acids in high yields. Esters such as methyl, ethyl, tert -butyl, and trityl esters as well as benzyl ethers, tert -butyl ethers, and N.

Benzyl ether (Bn)protecting group.
A non-hydrogenolytic mild deprotection strategy for stable benzyl protection of hydroxyl group is one of the long-cherished goals in multistep synthesis involving carbohydrates or compounds with multiple hydroxyl groups. A greener organo-photocatalytic method has been developed for mild and efficient visible light catalytic.

(PDF) VisibleLightMediated Oxidative Debenzylation Enables the Use of Benzyl Ethers as
As a protecting group Benzyl groups are occasionally employed as protecting groups in organic synthesis. Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.
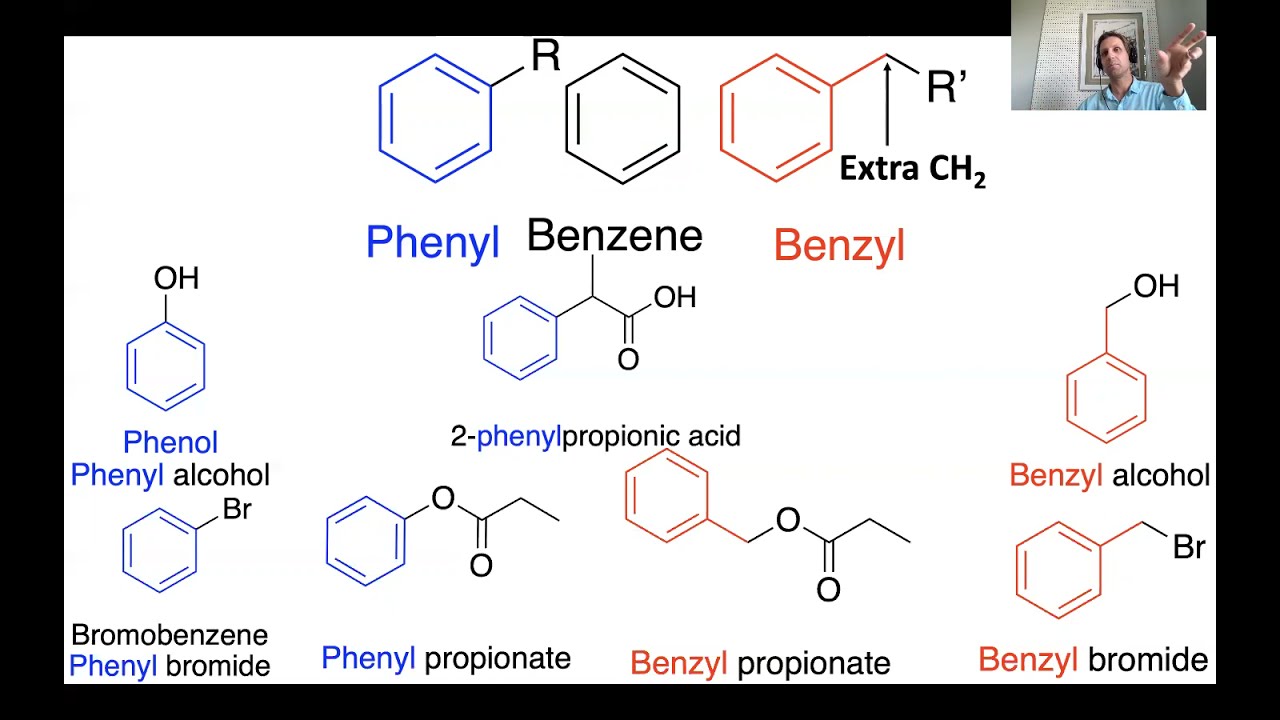
Phenyl vs Benzyl Groups YouTube
Use of NaH as base for the deprotonation is convenient, but when selective substitution is needed - for example, protection of one hydroxyl group in diols or selective protection of a more accessible group - mild bases such as Ag 2 O allow a more selective reaction.

Benzyl ether (Bn)protecting group.
The removal of benzyl groups from the nitrogen atom of an aziridine is a potentially useful component of protecting group chemistry. However, there are few examples of such a process. An example involving the removal of a monomethoxytrityl (mmTr) group under acidic conditions is shown in Equation (44) 〈93JOC7848〉.