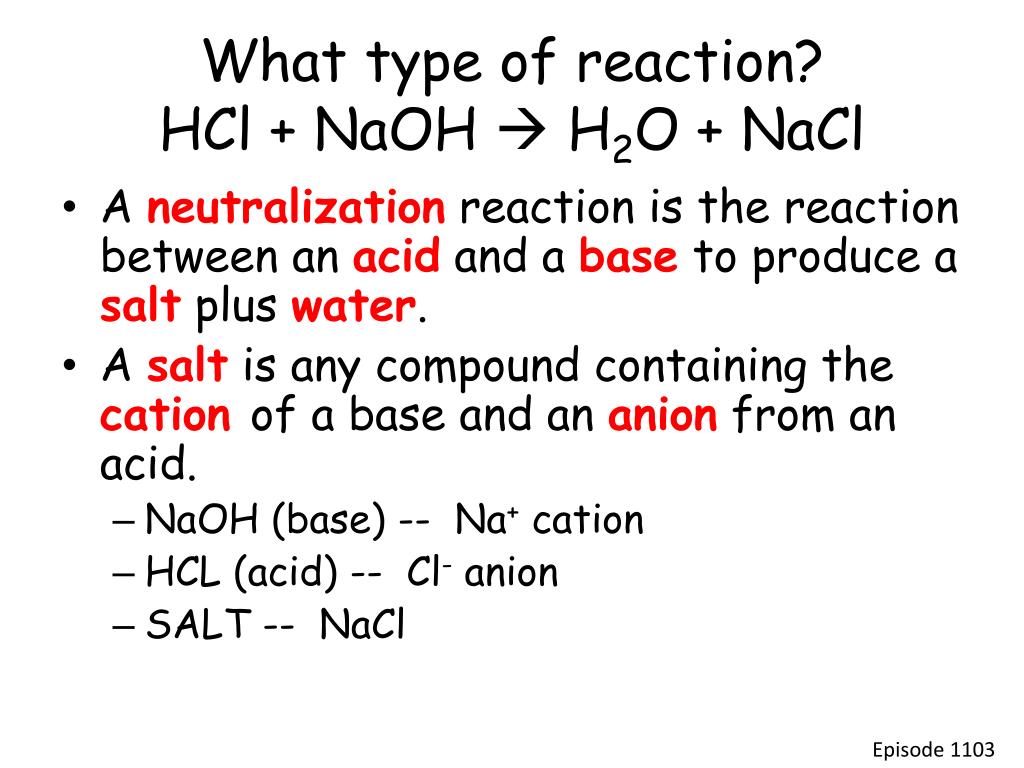
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The limiting reagent row will be highlighted in pink. Examples of complete chemical equations to balance: Fe + Cl 2 = FeCl 3 KMnO 4 + HCl = KCl + MnCl 2 + H 2 O + Cl 2
Al + hcl balanced equation
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O - Balanced equation | Chemical Equations online! Solved and balanced chemical equation Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O with completed products. Application for completing products and balancing equations.
Mgco3 + Hcl Balanced Equation With States Drawi
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Al 2 O 3 + 1 HCl = 1 Al 2 Cl 6 + 1 H 2 O For each element, we check if the number of atoms is balanced on both sides of the equation. Al is balanced: 2 atoms in reagents and 2 atoms in products. O is not balanced: 3 atoms in reagents and 1 atom in.

How to balance NaOH + HCL Balanced chemical equationStepbystep explained YouTube
Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 Al 2 O 3 + 1 HCl = 1 H 2 O + 1 AlCl 3 For each element, we check if the number of atoms is balanced on both sides of the equation. Al is not balanced: 2 atoms in reagents and 1 atom in products. In order to balance Al on both sides we: Multiply.
[Solved] Write the balanced equation for the reaction of Al(NO3) 3 and Na,... Course Hero
The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. Replace immutable groups in compounds to avoid ambiguity.

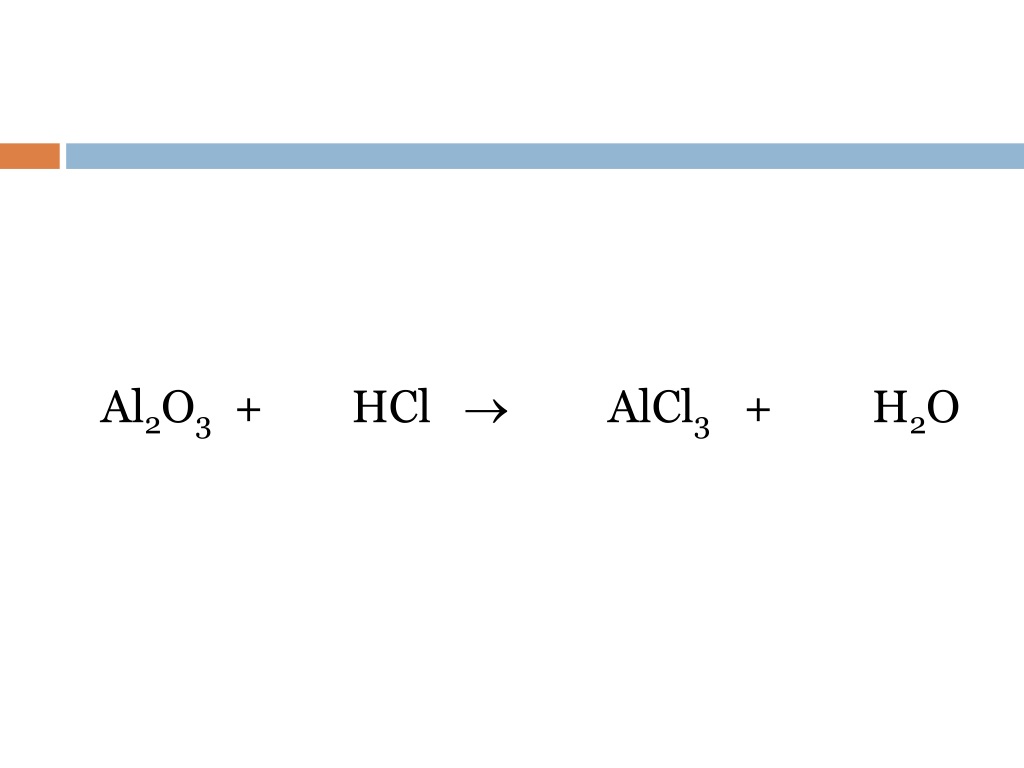
Balance this equation Al2O3+ HCl AlCl3 + H2O Science Metals and Nonmetals 12348429
HCl + Al2O3 = AlCl3 + H2O is a Double Displacement (Metathesis) reaction where six moles of Hydrogen Chloride [HCl] and one mole of Aluminum Oxide [Al 2 O 3] react to form two moles of Aluminum Chloride [AlCl 3] and three moles of Water [H 2 O] Show Chemical Structure Image Reaction Type Double Displacement (Metathesis) Exchange reaction Reactants

How to Write the Net Ionic Equation for Al2O3 + HCl Note Al2O3 should be (s). YouTube
Al2O3+HCl=AlCl3+H2O balance the chemical equation. Aluminium oxide and hydrogen chloride reaction My documentary 6.61K subscribers Subscribe 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4.

SOLVED Text From aluminum metal and oxygen Use the following data Aluminum oxide
Al2O3 | aluminium oxide | solid + HCl | hydrogen chloride | solid = AlCl3 | aluminium chloride | solid + H2O | water | solid | Temperature: temperature, Other Condition excess chlorine Introduce Detailed information about the equation Reaction conditions when applied Al2O3 + HCl Reaction process Al2O3 + HCl The result of the reaction Al2O3 + HCl

How to balance Al2O3+C=Al+CO2Chemical equation Al2O3+C=Al+CO2Al2O3+C=Al+CO2 balancedAl2O3+C
How to balance Al2O3+HCl=AlCl3+H2O|Chemical equation Al2O3+HCl=AlCl3+H2O| Al2O3+HCl=AlCl3+H2O Career Valley Institute 16K subscribers Subscribe 2.6K views 2 years ago How to.

Balancing Chemical Equations Worksheet With Answers H2 O2 H2o
Balanced Chemical Equation - Solution. Al 2 O 3 + 6 HCl → 2 AlCl 3 + 3 H 2 O. The coefficients show the number of particles (atoms or molecules), and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms.

How to Balance PbBr2 + HCl = HBr + PbCl2 and Type of Reaction) YouTube
How to Balance Al2O3 + HCl = AlCl3 + H2O (Aluminum Oxide + Hydrochloric Acid) Wayne Breslyn 727K subscribers Join Subscribe Subscribed 812 75K views 5 years ago In this video we'll balance.
PPT 30 Nov. 2010 Law of Conservation of Mass PowerPoint Presentation ID1532140
The HCl and Al2O3 reaction can be balanced by the hit and trial method according to the steps given below - HCl (aq) + Al2O3(s)= AlCl3(aq)+ H2O (l) As the reactant contains two aluminium atoms so two moles of the aluminium chloride must be present on the reactant side in order to balance the reaction. HCl (aq) + Al2O3(s)= 2AlCl3(aq)+ H2O (l)

SOLVEDAn electrolytic cell produces Al and O2 from Al2O3 at a rate of 5.00 kg per day (1.00 day
Chem Exam 3. Get a hint. Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with HCl according to the following balanced equation. Al2O3 (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2O (l) 72.9 g. 16.3 g.

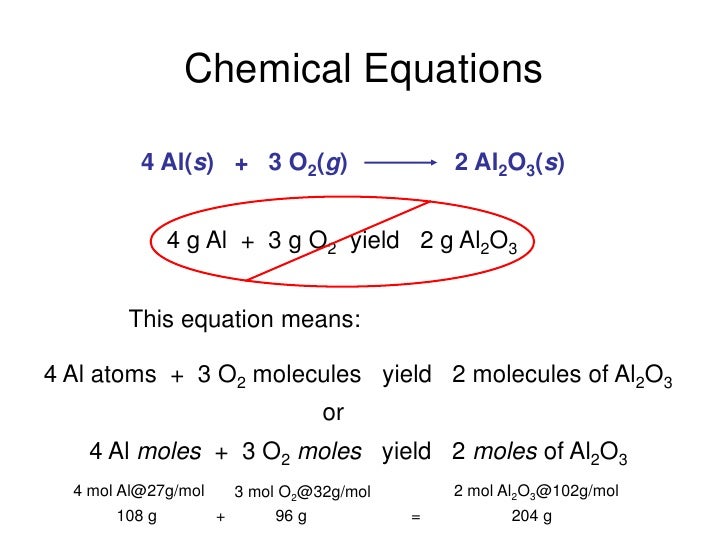
SOLVED Given the following thermochemical equations 4Al(s) 4Al(s) 301(g) 2Al,O3(s) 3MnOz(s
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Be sure to answer all parts. Balance each equation. Remember to include states of matter. Al2O3 (s) + HCl (aq) - AlCl3 (aq) + H2O (I) There are 3 steps to solve this one.
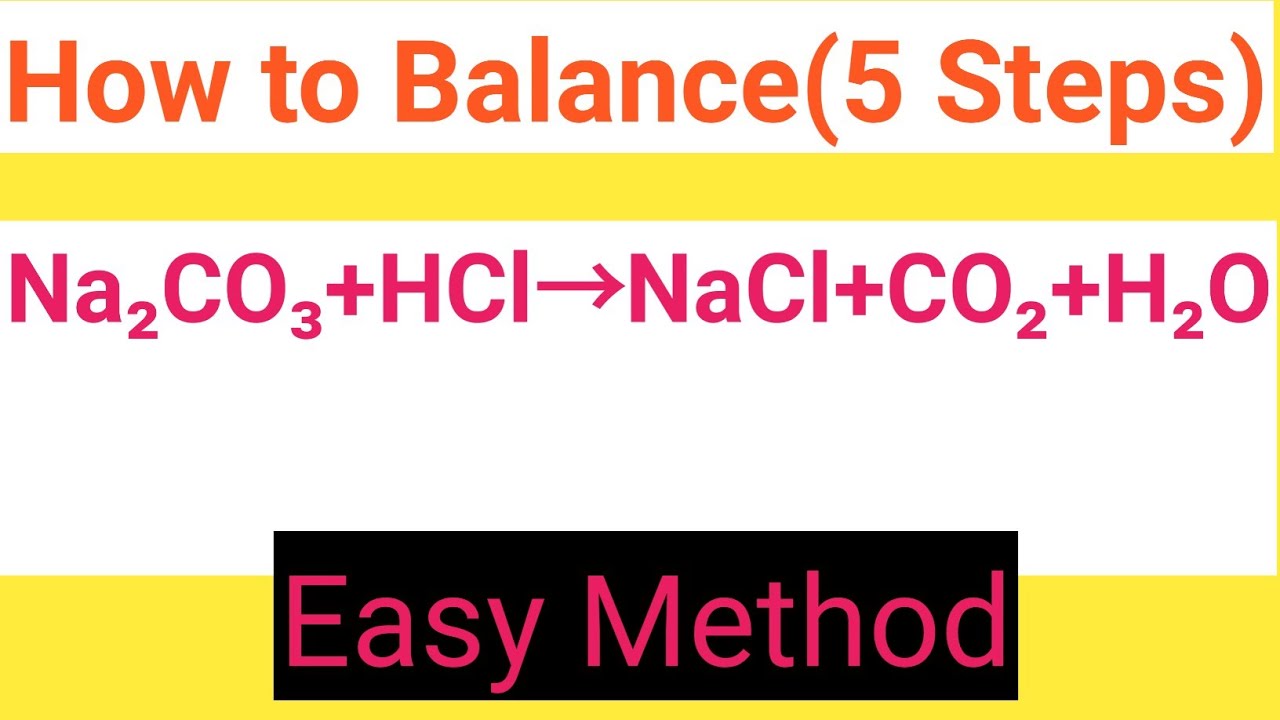
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate+Hydrochloric acid=Sodium chloride
1. F e+Al2O3→ 2. Cu+M gCl2 → 3. Cu+AlCl3→ 4. F e+HCl→ Q. Among Al2O3, SiO2, P 2O3, SO2 the correct order of acid strength is Al2O3 + HCl vrightarrow text

Find the mass of HCl that must react when starting with 23.2 grams of Al2O3, according
Balanced Chemical Equation Al 2 O 3 + 6 HCl → 2 AlCl 3 + 3 H 2 O ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Aluminum Oxide + Hydrogen Chloride = Aluminum Chloride + Water