
50 chemical equation with name and balance ti Brainly.in
Balance a chemical equation when given the unbalanced equation. Explain the role of the Law of Conservation of Mass in a chemical reaction. Even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not disappear, nor do new atoms appear to form the products.
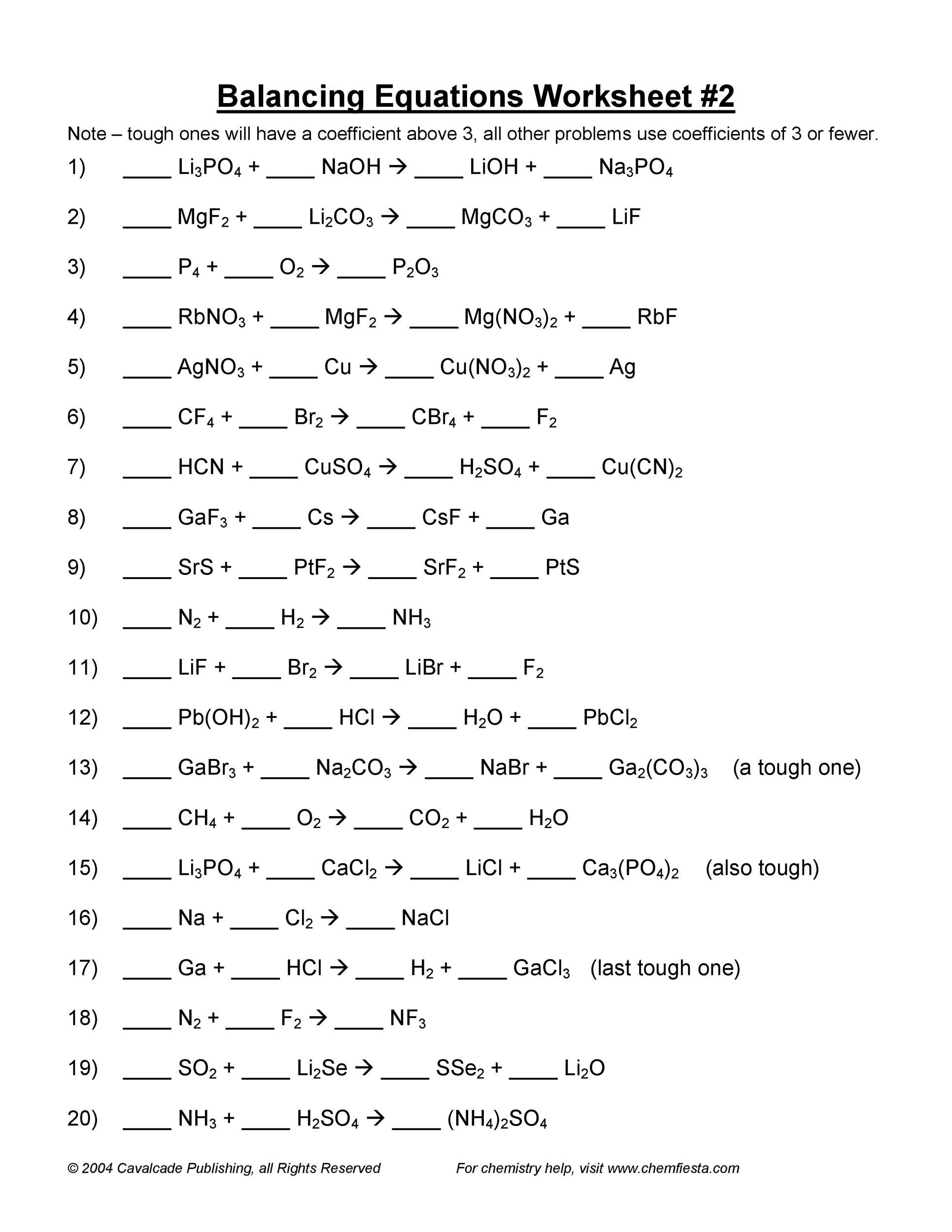
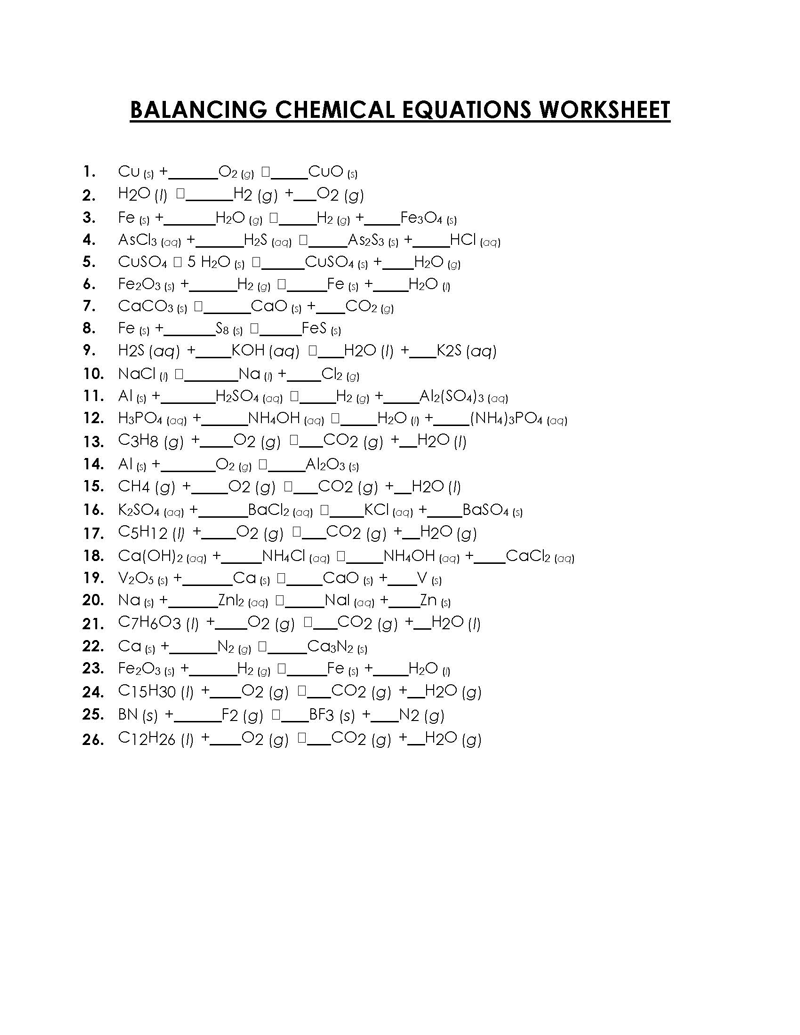
49 Balancing Chemical Equations Worksheets [with Answers]
Example of Balancing a Chemical Equation Let's illustrate this with an example by balancing this chemical equation: P 4 O 10 + H 2 O → H 3 PO 4 First, let's look at the element that appears least often. Notice that oxygen occurs twice on the left-hand side, so that is not a good element to start out with.

Balancing Chemical Equations — Overview & Examples Expii
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. It may be confirmed by simply summing the numbers of.
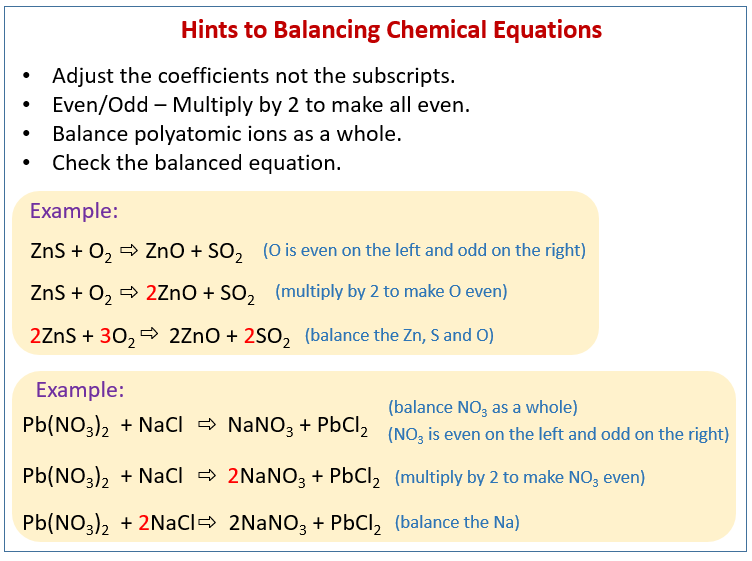
How to balance chemical equations (solutions, examples, videos)
1 What is a Chemical Equation? 2 Balancing Chemical Equations Worksheets 3 Why is it Important to Balance the Chemical Equations? 4 Balancing Equations Worksheets with Answers 5 What are Different Types of Chemical Equations? 6 Balancing Equations Practice Worksheet 7 How to Balance a Chemical Equation?
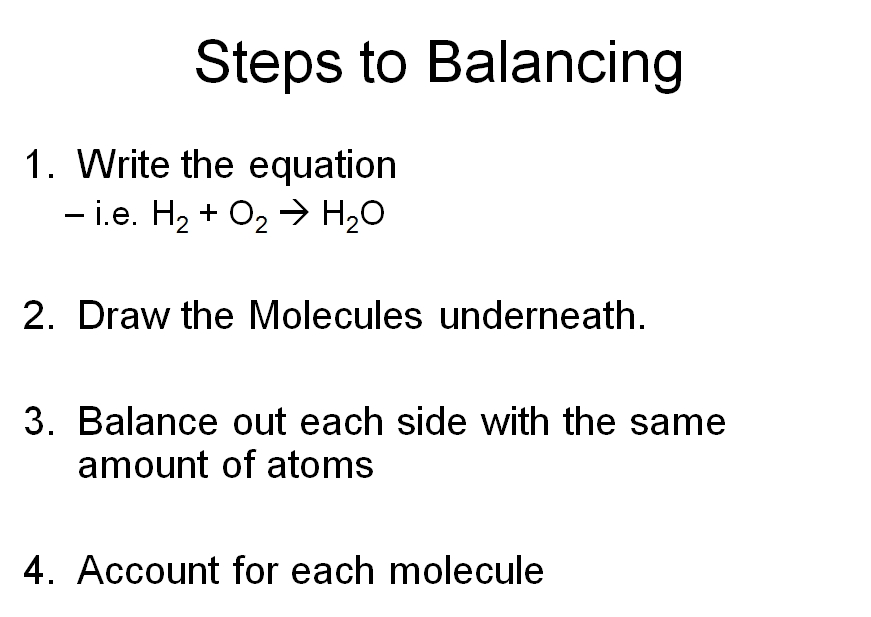
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE
CHEM 120: Fundamentals of Chemistry 4: Chemical Quantities and Reactions
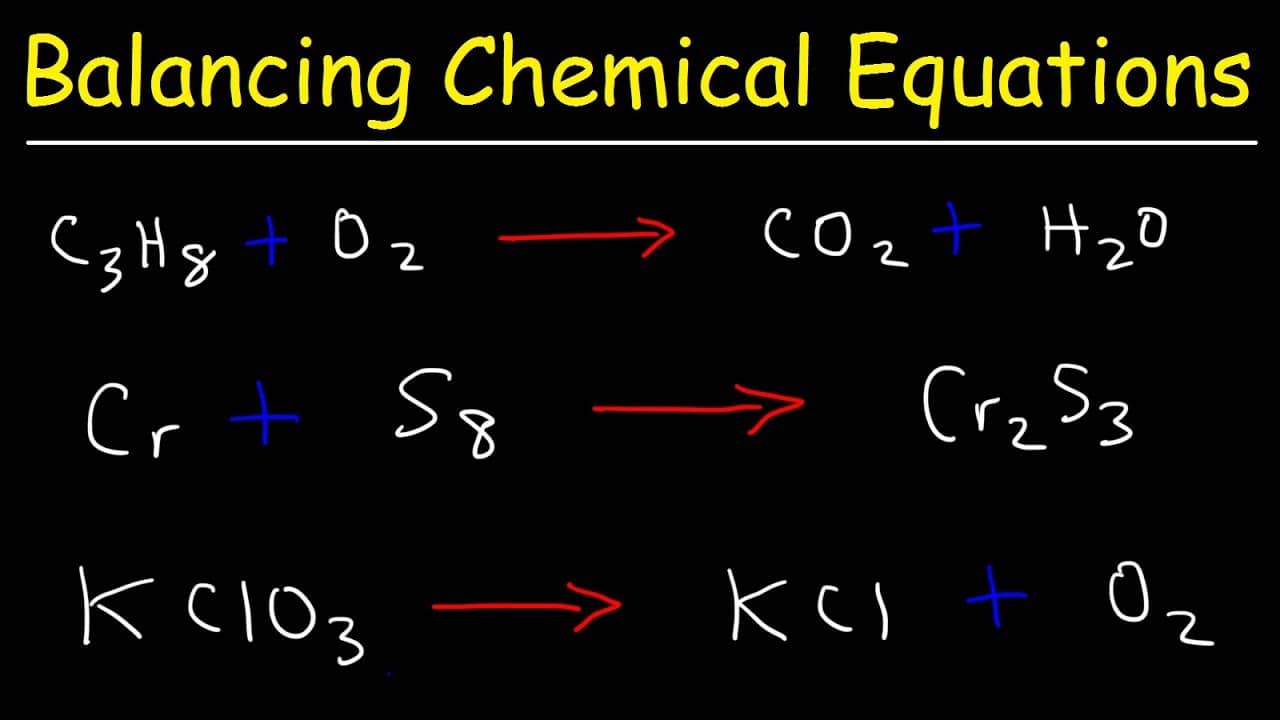
How to Balance Chemical Equations? Best Examples Get Education Bee
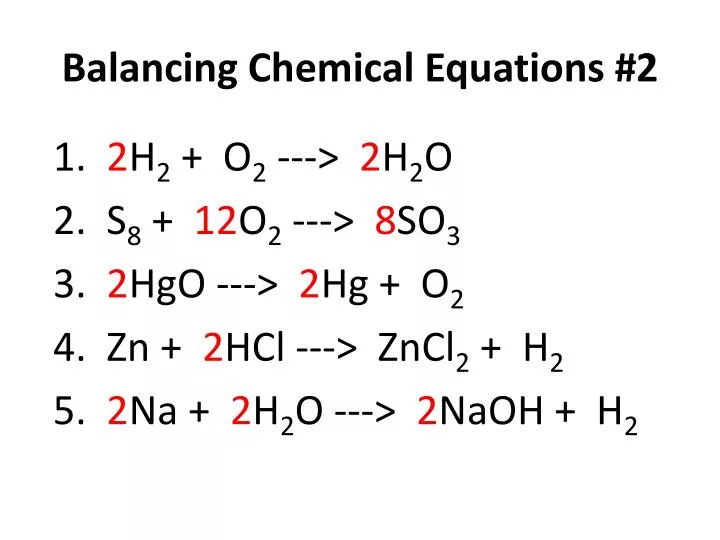
Here are 50 simple balanced chemical equations. 2Fe2O3+3C--->4Fe+3CO2 2)H2SO4+CaCO3--->CaSO4+H2CO3 3) 2H2+02--->2H20. What is the balanced chemical equation for magnesium hydroxide and hydrochloric acid? Medium. View solution > When balancing a chemical equation, why can you change coefficients, but not subscripts?.
.PNG)
Balancing Chemical Equations Presentation Chemistry
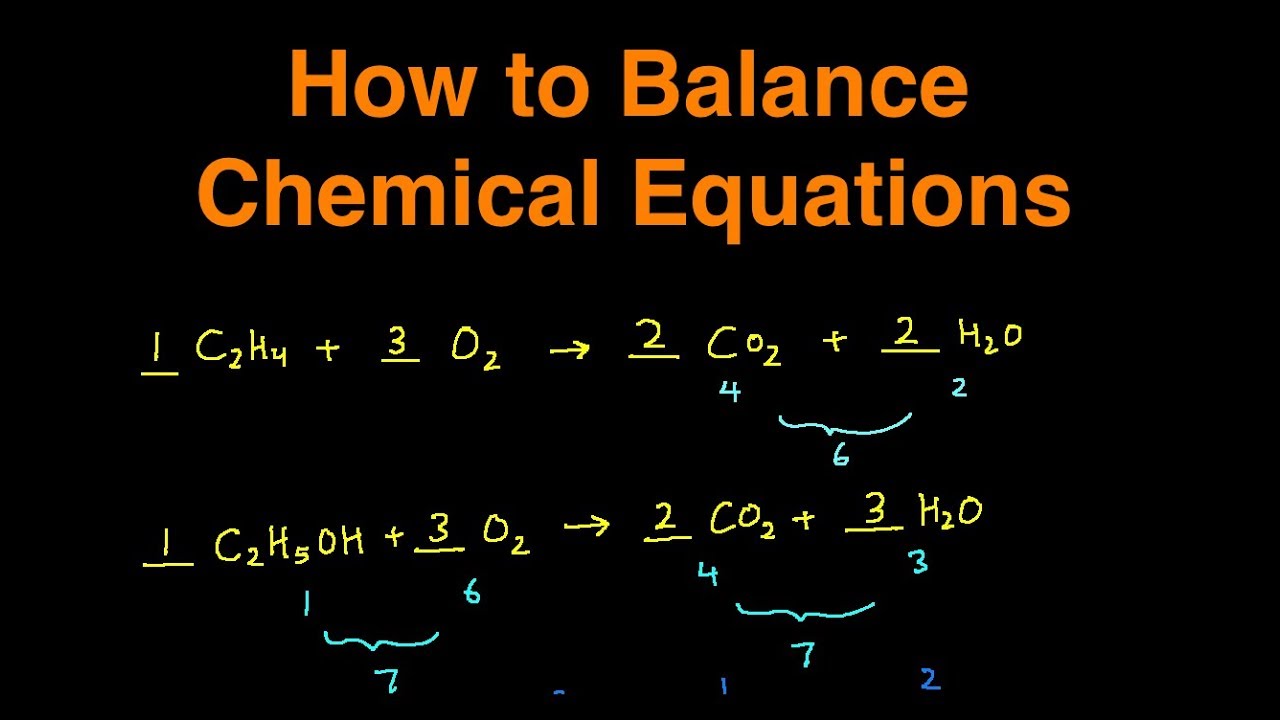
Write Down Number of Atoms. The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow: Fe + O 2 → Fe 2 O 3. To do this, keep in mind a subscript indicates the number of atoms. For example, O 2 has 2 atoms of oxygen.

49 Balancing Chemical Equations Worksheets [with Answers]
The chemical formula of propane is C 3 H 8. It burns with oxygen (O 2) to form carbon dioxide (CO 2) and water (H 2 O) The unbalanced chemical equation can be written as C3H8 + O2 → CO2 + H2O Step 2 The total number of atoms of each element on the reactant side and the product side must be compared.
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Answers
Chemistry Examples. Step-by-Step Examples. Chemistry. Chemical Equations and Reactions. Balance. Li + H2O → LiOH + H2 L i + H 2 O → L i O H + H 2. The law of conservation of mass states that matter cannot be created nor destroyed. There are no more or less atoms at the end of a chemical reaction than there were at the beginning.
How to Balance Chemical Equations Step by Step Explanation with Examples & Practice Problems
Balancing Chemical Equations in Five Easy StepsBalancing chemical equations is a core skill in chemistry. In this video you'll learn the basics for balancin.

50 balanced chemical equations Brainly.in
For example, let's look at the following chemical equation - Zinc + Sulphuric acid → Zinc sulfate + Hydrogen This is further simplified as - Zn + H 2 SO 4 → ZnSO 4 + H 2 Now, let us look at the number of atoms of each element on LHS and RHS - As you can see, the number of atoms of each element on LHS and RHS are the same.

How to Balance Chemical Equations 10 Steps (with Pictures)
General Chemistry Map: Chemistry - The Central Science (Brown et al.) 3: Stoichiometry- Chemical Formulas and Equations 3.1: Chemical Equations
45 Free Balancing Chemical Equations Worksheets (PDF)
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the.

50 Examples Of Balanced Chemical Equations With Answers Pdf Tessshebaylo
Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. Indicate the states of matter of the reactants and products. Use (g) for gaseous substances. Use (s) for solids. Use (l) for liquids. Use (aq) for species in solution in water.
HelpWork balancing chemistry equations
Balancing chemical equations 1 Google Classroom Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O Note: All reactants and products require a coefficient of at least one. Stuck? Review related articles/videos or use a hint. Report a problem Do 4 problems

How to balance chemical equations
Here are examples of balanced equations you can review or use for homework. Note that if you have "1" of something, it does not get a coefficient or subscript. The word equations for a few of these reactions have been provided, though most likely you'll be asked to provide only the standard chemical equations .