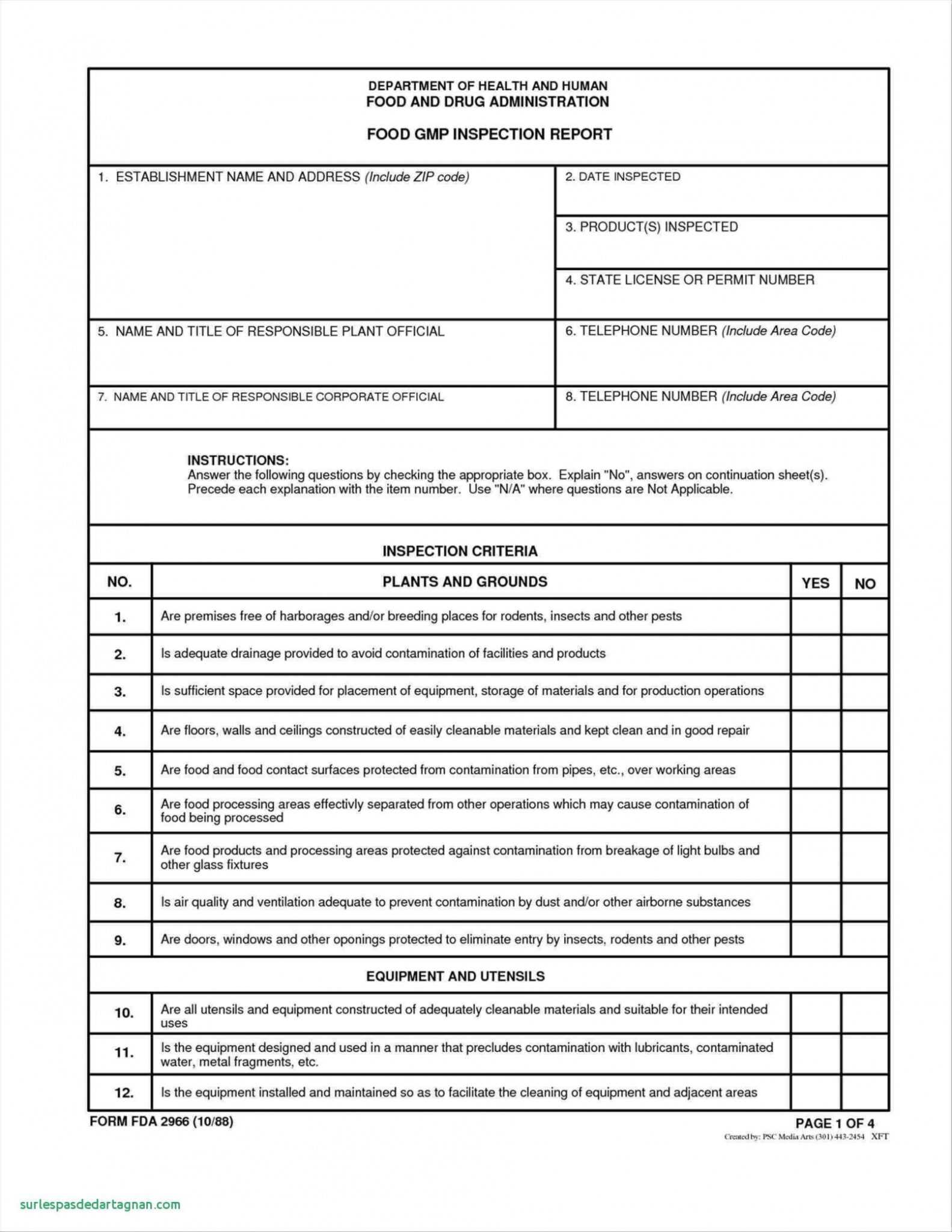
Gmp Audit Report Template
Safety and Security - Pharmaceutical manufacturing is strictly regulated, and intensive safety and security measures are paramount. This section of the GMP audit template covers these safety and security issues. Security isn't merely in place to keep personnel and employees from accessing the incorrect facility controls.

Gmp Audit Report Template
Lumiform is an inspection and software app that can help your company with GMP planning and documentation. Carry out paperless GMP controls with any mobile device. Get notified about routine checks using automatic notifications. Use free digital GMP checklist templates and customize them to your company.

Gmp Audit Report Template
Disclaimer. This GMP audit checklist is intended to aid in the systematic audit of a facility that manufactures drug components or finished products. The adequacy of any procedures is subject to the interpretation of the auditor. Therefore, ISPE and the GMP Institute accept no liability for any subsequent regulatory observations or actions stemming from the use of this audit checklist.
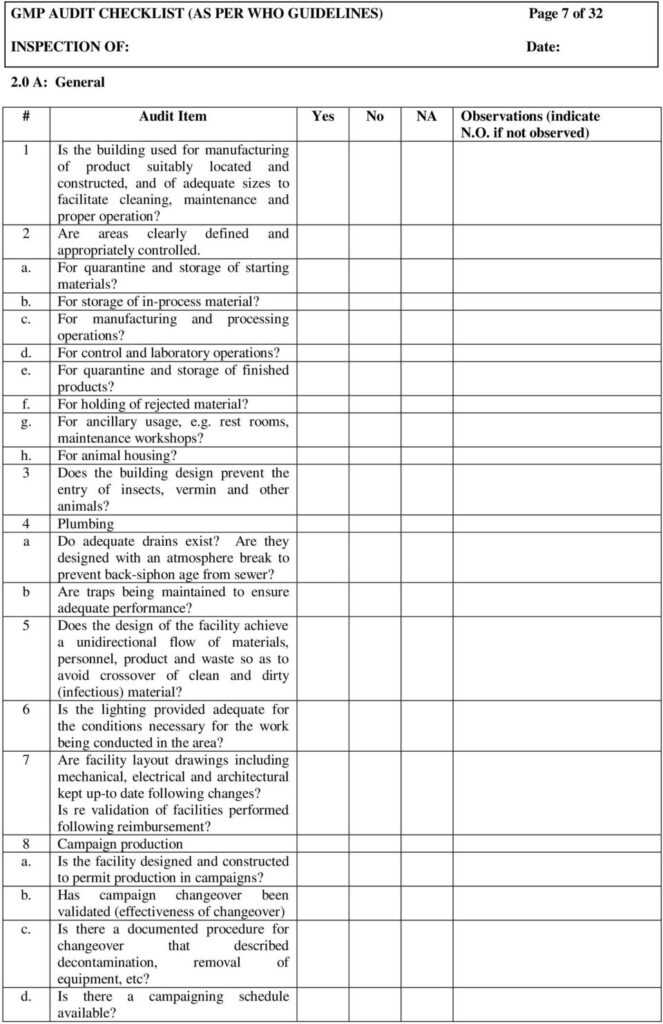
Gmp Audit Checklist (As Per Who Guidelines) Page 1 Of 32 In Gmp Audit Report Template Best
Draft a report Follow up with management That's a vague list, so let's ensure we're on the same page and go into more detail. Define the scope of the audit The first step in any GMP audit is to define the scope of the inspection.

GMP AUDIT CHECKLIST (AS PER WHO GUIDELINES) M A N O X B L O G
In addition, GxPorbit offers a comprehensive range of GMP audit services to help pharmaceutical firms prepare for a GMP audit. This includes a GMP audit checklist, which is designed to ensure that all relevant areas of the facility are inspected, as well as an audit report template that can be customized to meet the specific needs of each company.

gmp audit checklist Archives PROFESSIONAL TEMPLATES PROFESSIONAL TEMPLATES
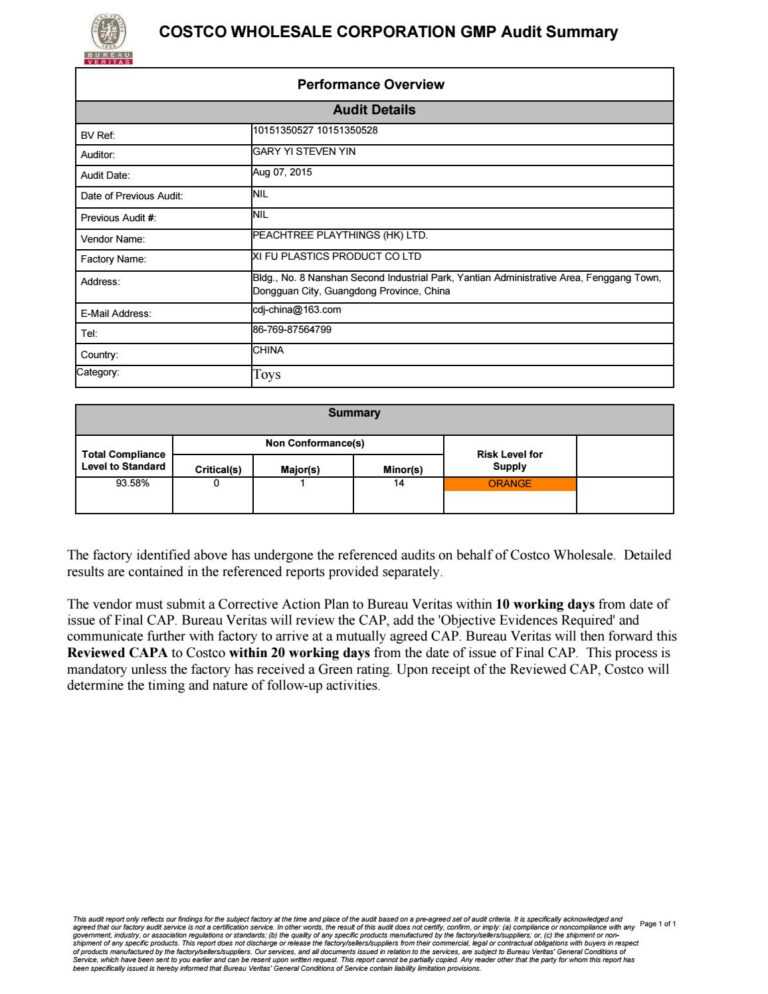
Good Manufacturing Practices - Audit Report Form (FRM-0211) - Instructions. Good Manufacturing Practices - Request for Inspection of a Foreign Site Form (FRM-0213) Good Manufacturing Practices - Foreign Site Inspection Services Agreement Form (FRM-0214) Date modified: 2023-08-28. Health Canada forms related to good manufacturing practices (GMP.
Gmp Audit Report Template
Likewise, all observations on the audit report should be fair and balanced. It is also advisable to use non-inflammatory or non-derogatory language. Do not forget that a Good Manufacturing Practices audit aims to make an organization perform better. Accomplish Your Audit Report promptly; Drafting the audit report could be time-consuming.

Gmp Audit Report Template (11) TEMPLATES EXAMPLE TEMPLATES EXAMPLE Internal Audit, Report
This global management plan audit checklist is a standard guide used to compare a company's manufacturing and business management practices against the standard practices of a pharmaceutical industry, in compliance with government regulations.
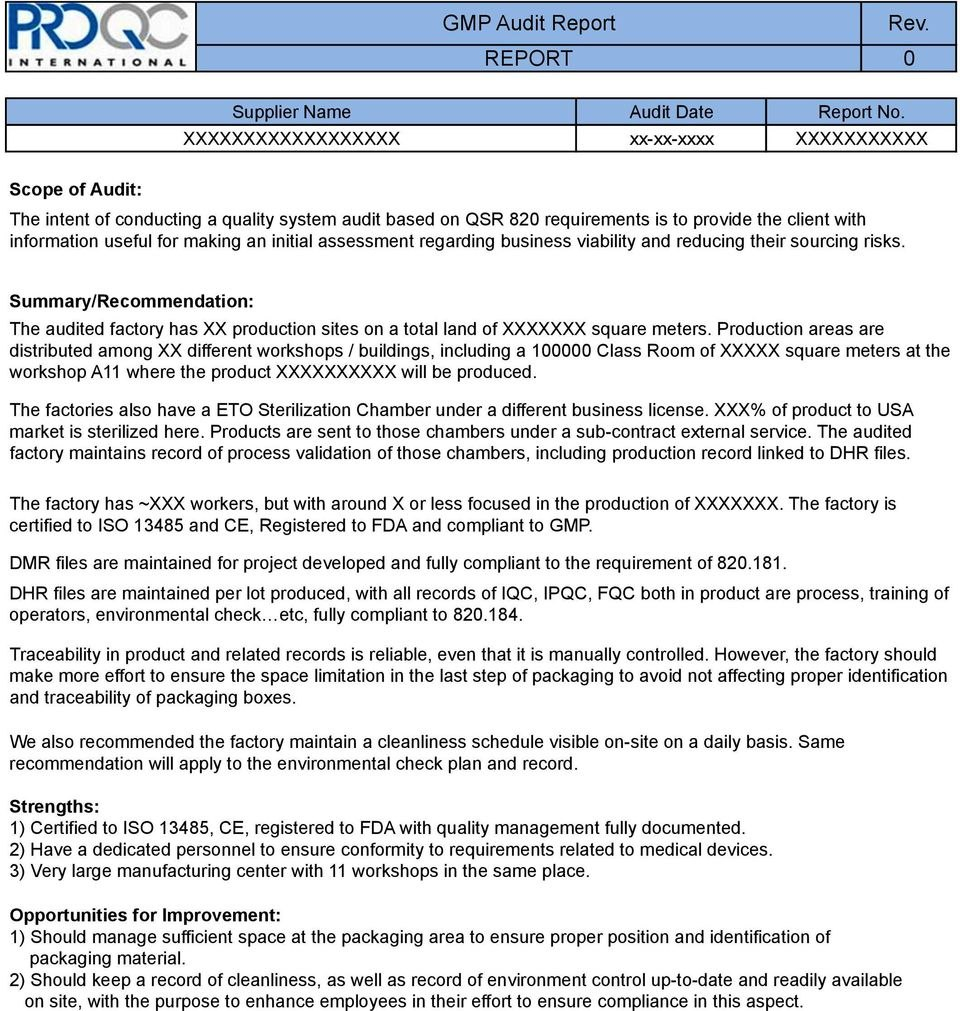
Gmp Audit Report Template 10+ Examples of Professional Templates Ideas
Audit Report With GMP Questionnaire (Template) | PDF | Quality Assurance | Verification And Validation 2. AUDITSCOPEANDOBJECTIVES 7 2.1. Background..7 3. INTRODUCTION&COMPANYOVERVIEW 9 3.1.

Gmp Audit Report Template
GMP Audit Checklist. Maintain compliance and product quality with our comprehensive Good Manufacturing Practices (GMP) Audit Checklist. This free PDF template is designed to help you systematically assess and verify adherence to GMP standards within your manufacturing facility. By conducting regular GMP audits, businesses can ensure the safety.
Gmp Audit Report Template
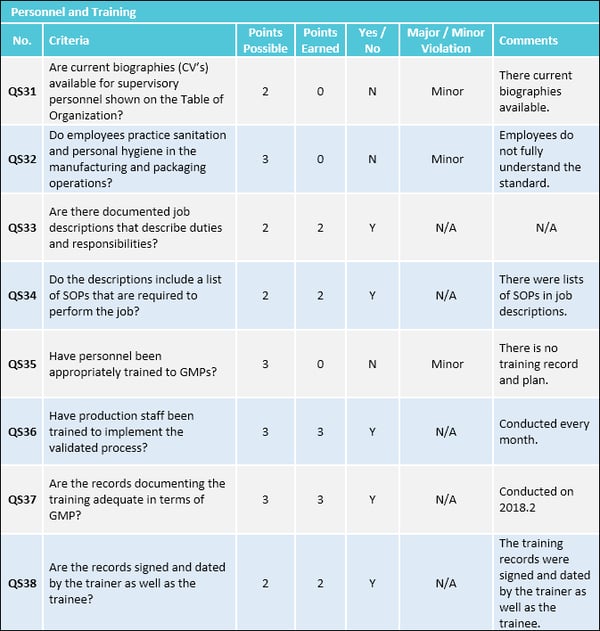
Good Manufacturing Practices - Audit Report Form (FRM-0211) v2 11 Sanitation [C.02.008] (1) Every person who fabricates or packages/labels a drug shall have, in writing, minimum requirements for the health and the hygienic behaviour and clothing of personnel to ensure the clean and sanitary fabrication and packaging/labelling of the drug.

Gmp Audit Report Template (5) TEMPLATES EXAMPLE TEMPLATES EXAMPLE Report template
Applicants may submit a corporate or consultant audit report, using the Audit Report Form, as evidence to establish the compliance of a foreign site with Division 2 (Good Manufacturing Practices) of the Food and Drug Regulations (FDR), so long as the criteria outlined in the guidance " Guidance: How to demonstrate foreign building compliance wit.

Gmp Audit Report. * Example Report * Pdf Within Gmp Audit Report Template
Audit Template Report GMP Registration Annual Audit Section 0. Visit Summary (0) Opening meeting attendees: Name and Title: (Max Score: 0) Answer: Employees who assisted during the audit: (Max Score: 0) Answer: Closing meeting attendess: Name and Title: (Max Score: 0) Answer: Section 1. 21 CFR 111: Subpart B: Personnel (0)
Gmp Audit Report Template Best Professional Templates
Technical Information. Exhibitions & Sponsoring. Members Area. ECA Academy. Guidelines. GMP Guidelines. Guidelines Detail. The following guideline can be ordered through the address listed in the "Source/Publisher"-category. In cases in which you can order through the Internet we have established a hyperlink.

Gmp Audit Report Template Sample Design Templates
The GMP audit schedule acts as an initial plan, with the company amending the schedule to take into account activities which may impact on the effectiveness of the quality management system, such as the results of previous audits, significant changes to working practices, the appointment or removal of key personnel etc.
Gmp Audit Report Template
An audit is a systematic and independent review to verify compliance, suitability and/or data integrity. Audits may assess: systems, processes, procedures, facilities, products, records and/or data for compliance with policies, standards, procedures, guidelines, regulations or regulatory submissions. The Documentation Database: