
BH3 Lewis Structure YouTube
Figure 1.2j BH3 molecule Lewis structure. BF 3 molecule: Even though all the atoms do have a chance to get octets in the structure of BF3, the actual structure of BF3 keeps the incomplete octet. Applying the FC guideline explains why the first structure is the better choice. Similar examples include BeF2 and AlCl3.

Chemfig How Can I Draw A Lewis Structure Tex Latex Stack Exchange Hot
Lewis structure of BH3 contains three single bonds between the Boron (B) atom and each Hydrogen (H) atom. The Boron atom (B) is at the center and it is surrounded by 3 Hydrogen atoms (H). Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me.

BH3 Lewis Structure (Boron Trihydride) YouTube
In the BH 3 Lewis structure, there are three single bonds around the boron atom, with three hydrogen atoms attached to it, and none of the atoms has a lone pair. BH3 Lewis Structure - How to Draw the Lewis Structure for BH3 Watch on Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms External links Steps

Incredible Is Bh3 Polar Or Nonpolar References
Lewis Dot Structure of BH3 (Boron Hydride) - YouTube 0:00 / 1:11 Lewis Dot Structure of BH3 (Boron Hydride) kentchemistry.com 24.8K subscribers 58K views 11 years ago I quickly take you.

BH3 Molecular Geometry YouTube
Steps of drawing BH3 lewis structure Step 1: Find the total valence electrons in BH3 molecule In order to find the total valence electrons in BH3 molecule, first of all you should know the valence electrons present in boron atom as well as hydrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Lewis Structure of Borane BH3 YouTube
Borane (BH3) lewis structure is made up of three B-H bonds, with boron (B) in a central position and all three hydrogens (H) as outer atoms in the lewis diagram. The lewis structure of BH3 contains a total of 3 bond pairs and 0 lone pairs. The drawing of the BH3 lewis's structure is very easy and simple. Let's see how to do it.

BF3 Lewis Structure, Molecular Geometry, and Hybridization
Thus, boron commonly forms three bonds, BH 3 , with a total of six electrons in the outermost shell. This also results in some anomalous properties for boron compounds because they are kind of "short of electrons". It should be thus noted that covalent bonding between non-metals can occur to form compounds with less than an octet on each atom.

Lewis structure/sharing of electrons of bh3 Brainly.ph
Home Bookshelves Organic Chemistry Organic Chemistry I (Liu) 1: Basic Concepts in Chemical Bonding and Organic Molecules

BH3 Lewis Structure How to Draw the Lewis Structure for BH3 YouTube
The Lewis structure of BH3 presents that B has three valence electron, and H has only one valence electrons. Steps to draw the Lewis Structure of BH3 Follow the below-mentioned steps to draw the Lewis structure of BH3 Step 1: Let's count the valence electrons in borane molecule first.

The structure of BH3 is Chemistry Questions
BH 3 is a trigonal planar molecule with D 3h symmetry. The experimentally determined B-H bond length is 119 pm. [5] In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: [6] BX 3 +BH 4− → HBX 3− + (BH 3) (X=F, Cl, Br, I)
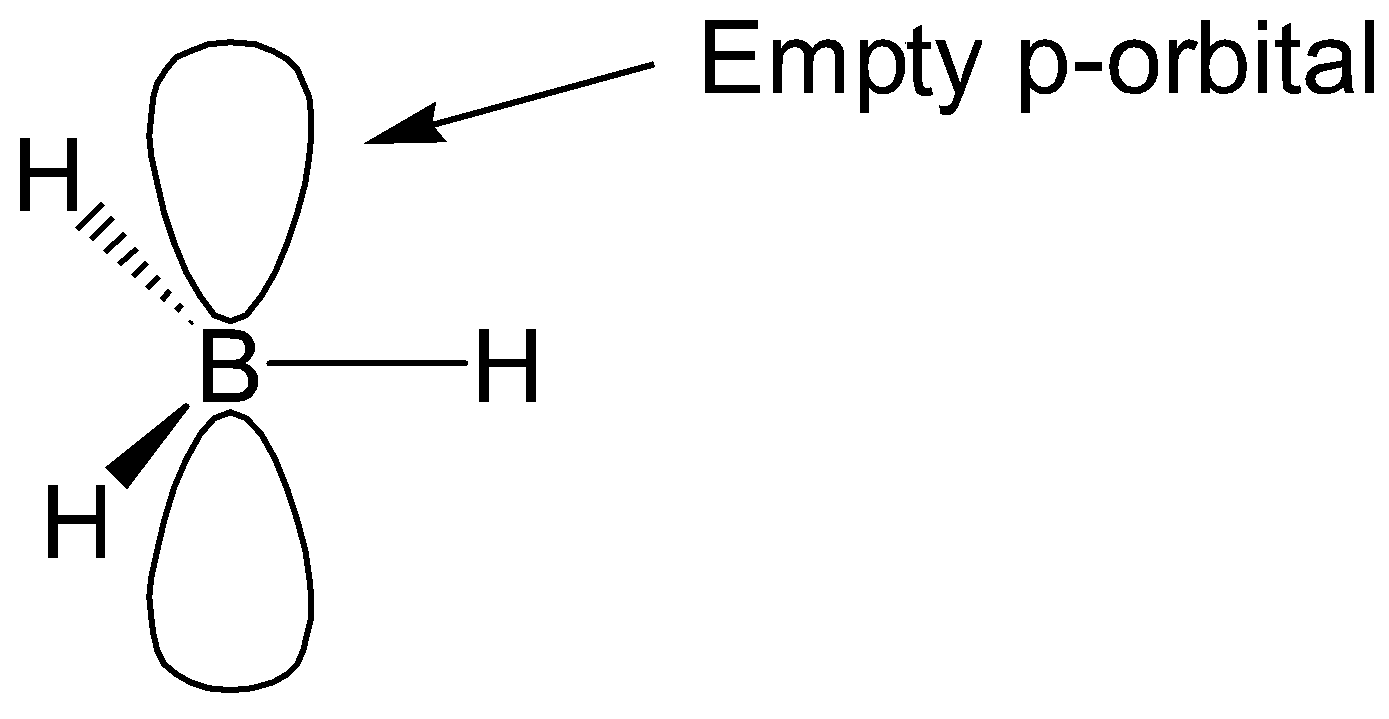
Lewis Electron Dot Structure For Boron
The molecule adopts a structure similar to that of ethane, with which it is isoelectronic. The B−N distance is 1.58 (2) Å. The B−H and N−H distances are 1.15 and 0.96 Å, respectively. Its similarity to ethane is tenuous since ammonia borane is a solid and ethane is a gas: their melting points differing by 284 °C.
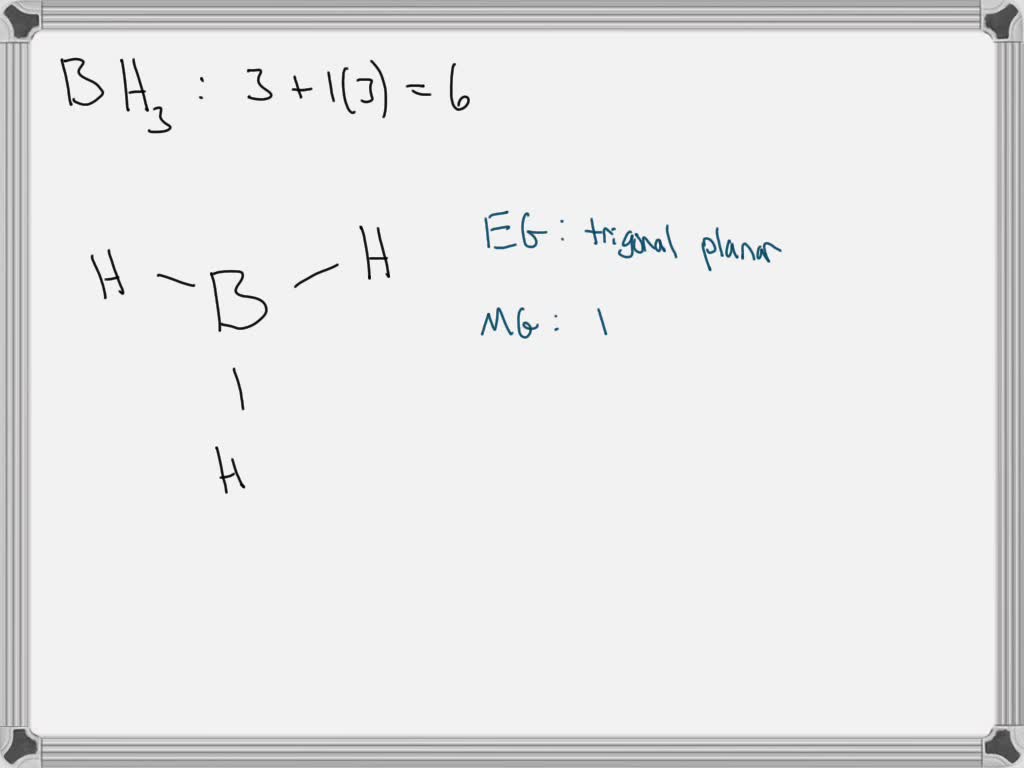
SOLVED Question 27 For the molecule given bclow BH3 Draw the Lewis
A step-by-step explanation of how to draw the BH3 Lewis Dot Structure (Boron Trihydride).There are only 6 valence electrons in the Lewis structure for BH3.Th.
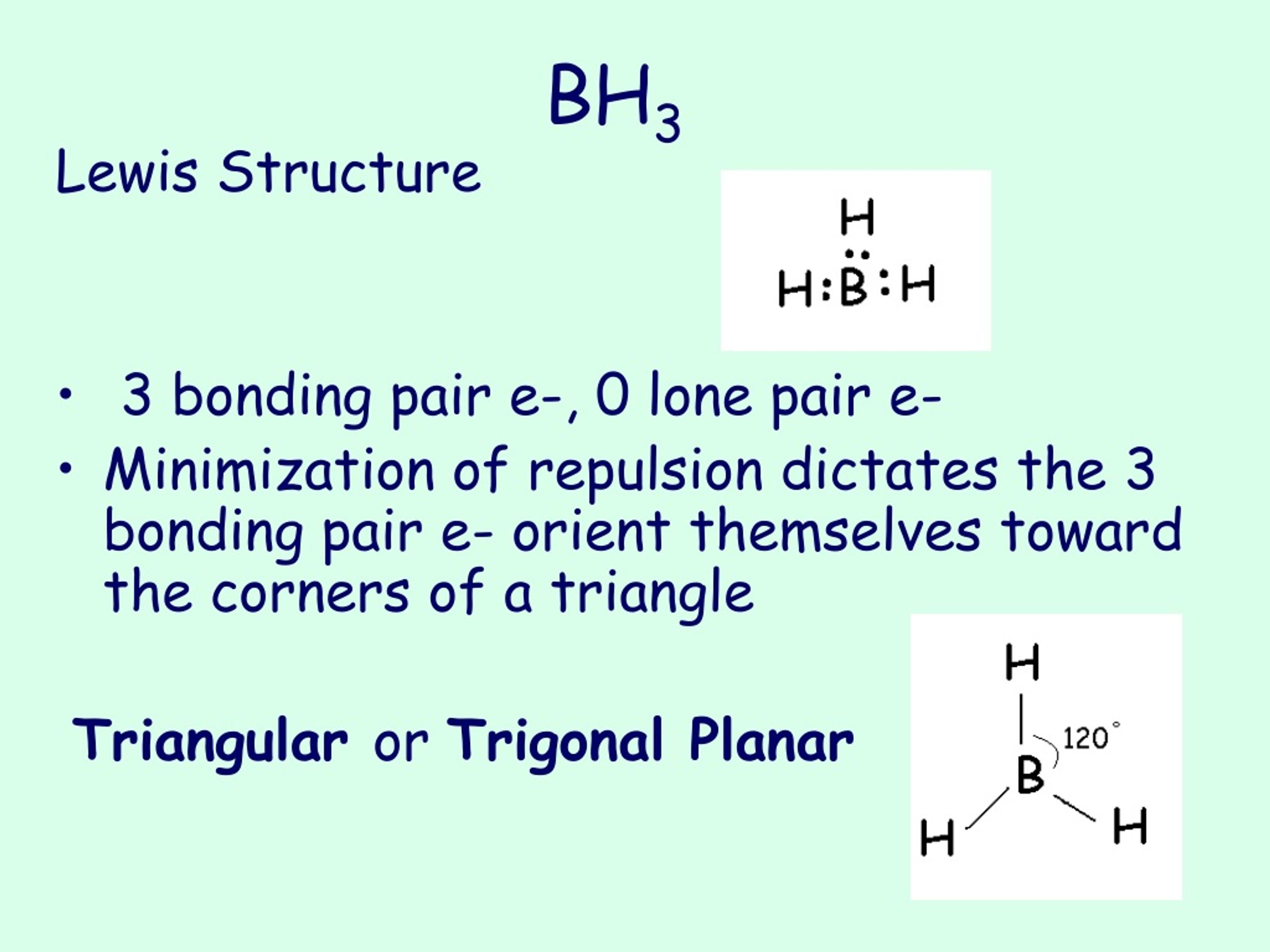
PPT Molecular Geometry PowerPoint Presentation, free download ID343710
The Lewis structure of BH3 shows that it is a trigonal planar molecule with a central boron atom and three hydrogen atoms surrounding it. BH3 is a highly reactive molecule and is often used as a Lewis acid in chemical reactions. Understanding the Lewis structure of BH3 helps in predicting its chemical behavior and reactivity. BH3 Lewis Structure

Lewis Dot Structure Of Nh3
Borane (BH 3) is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane (BH3). There are only three bonds around boron atom and no lone pairs on boron atom.
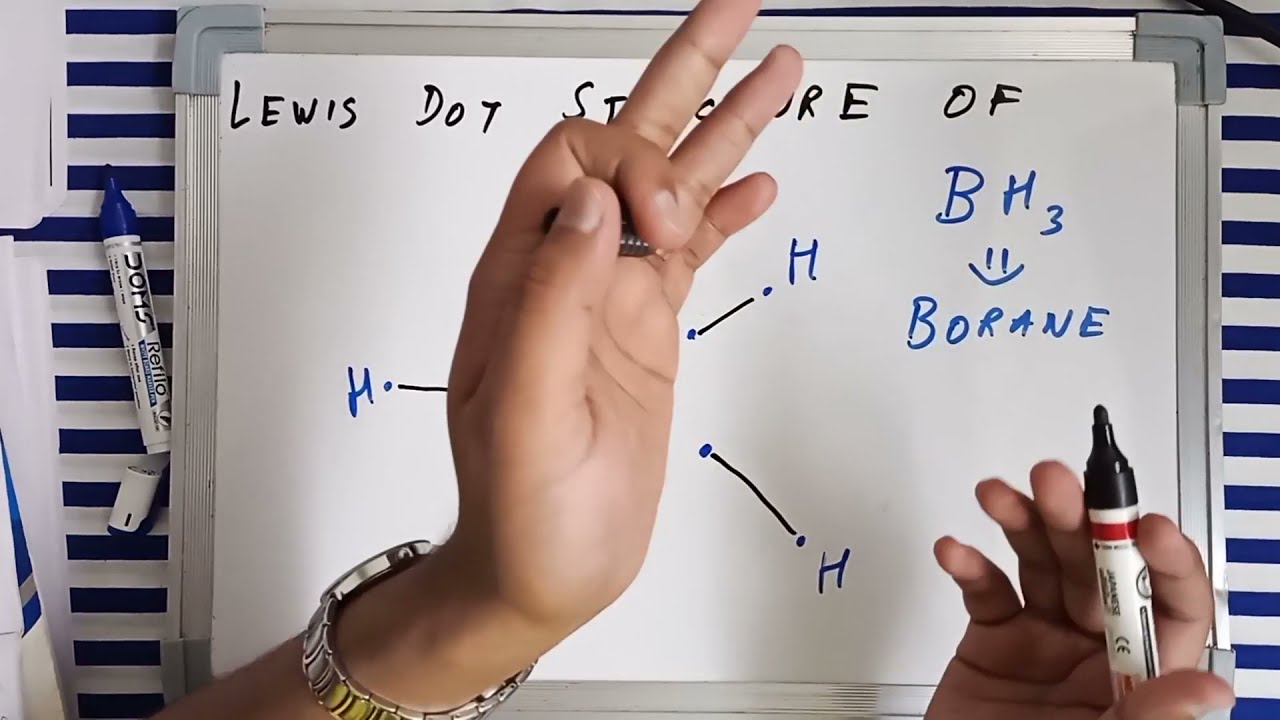
Lewis dot structure and hybridisation of BH3 Borane lewis structure
The Lewis base donates an electron pair to form a covalent bond with the Lewis acid (Fig. 4.1.2). A covalent bond formed in a Lewis acid-base reaction is usually called a dative bond because both electrons in the covalent bond come from a single partner. In a "conventional" covalent bond both partners contribute one electron to the covalent bond.

Bh3 ün lewis nokta gosteriminde Bor un elektronlari toplami bağ sonunda
Boron trihydride has a stable sextet. It is extremely reactive - even reacting with itself if there is nothing else.0:00 Lewis structures1:49 Shape and angl.