ShowMe beryllium bohr model
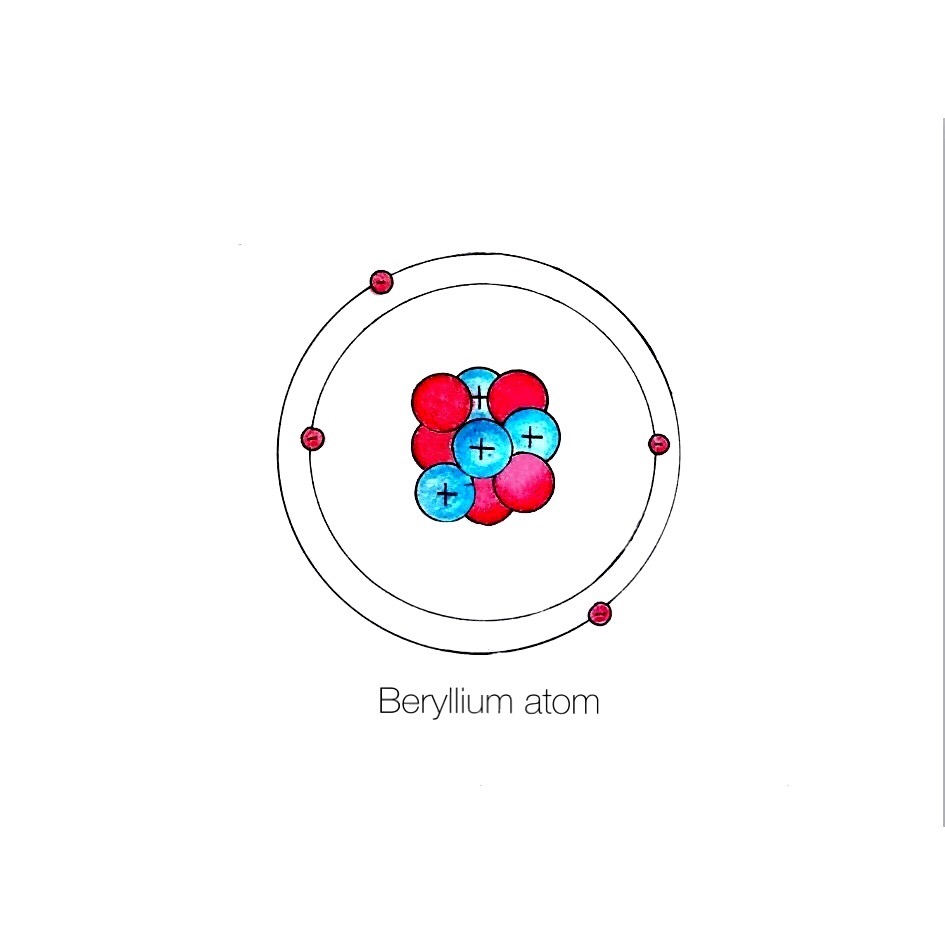
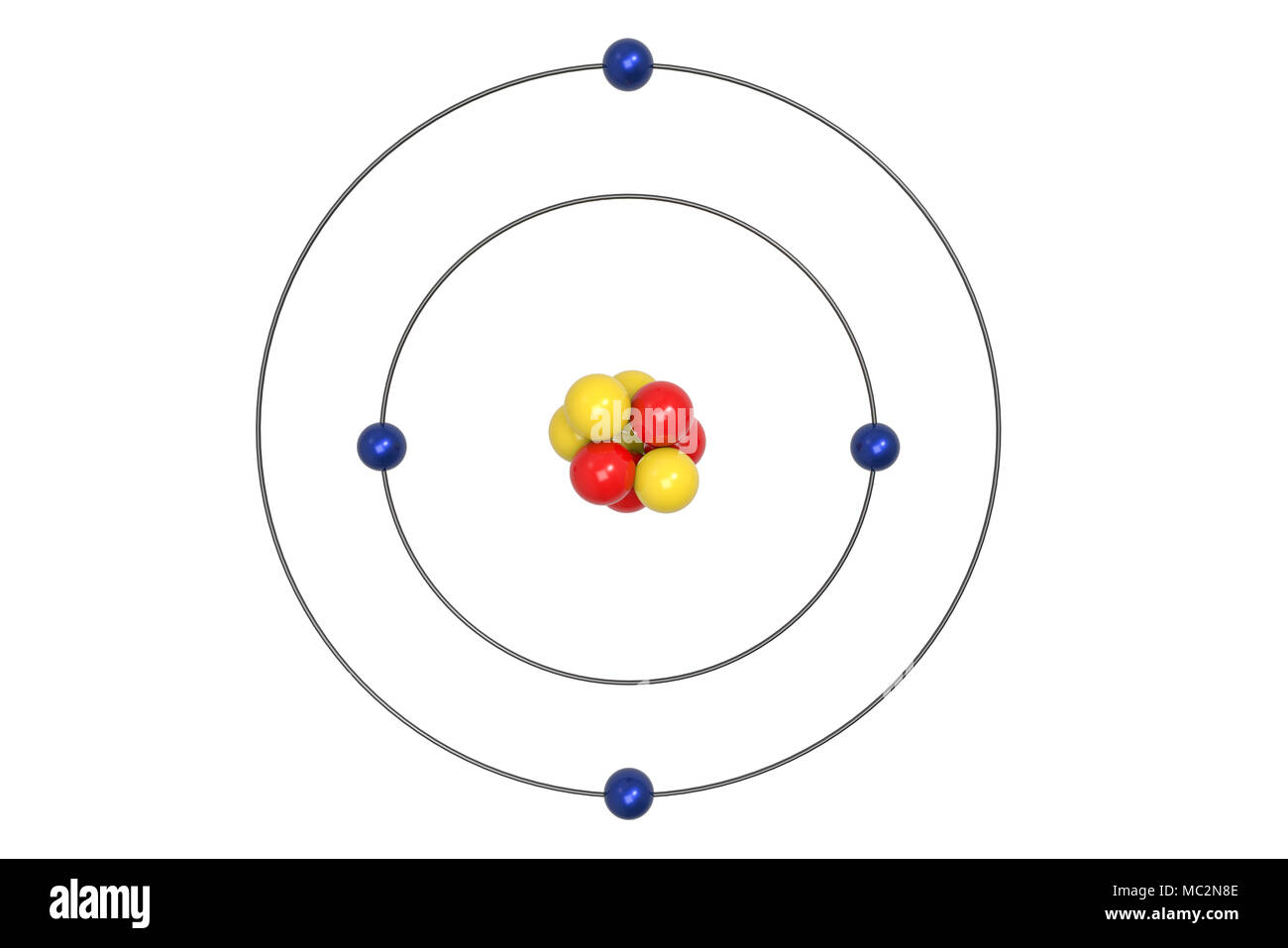
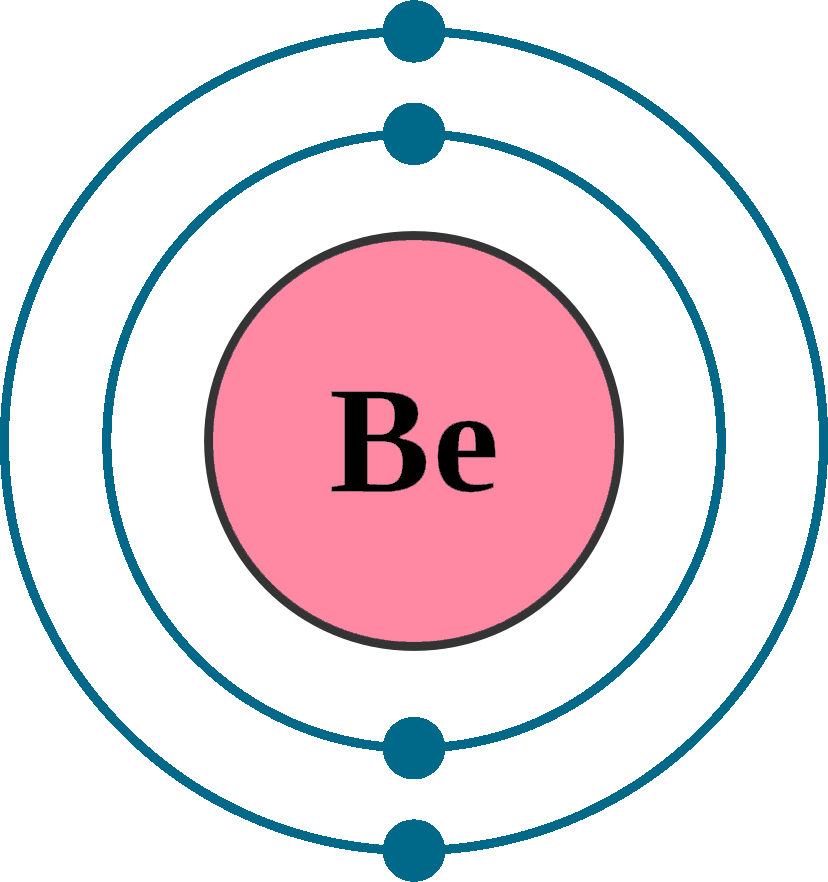
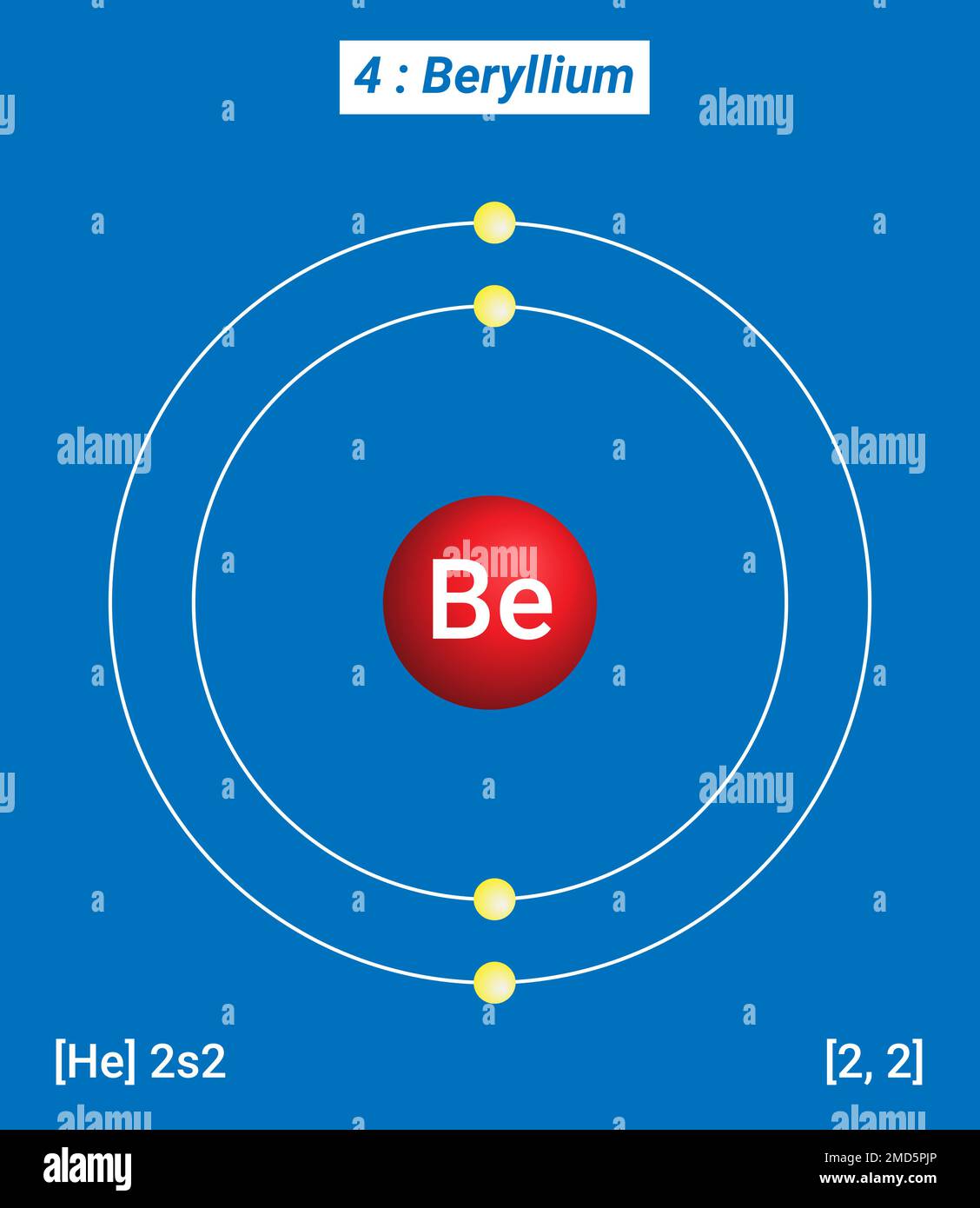
The Bohr Model of Beryllium (Be) has a nucleus that contains 5 neutrons and 4 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Beryllium contains 2 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Beryllium (Be)?
Beryllium Bohr Diagram exatin.info
Electrons arrangement or Bohr model: 2, 2: Electronic configuration [He] 2s 2: Atomic radius: 153 picometers (van der Waals radius) Valence electrons: 2: 1st Ionization energy: 9.323 eV:. You have already seen the Bohr model of Beryllium atom in the above table and you have seen that the number of orbits or shells in sodium is 2.
Bohr Diagram For Beryllium General Wiring Diagram
enhance student understanding of atomic structure. A Bohr model, more correctly a deBroglie-Bohr model, is used here to calculate the total electronic energies of atoms and ions containing up to four electrons. The Bohr model for the hydrogen atom is the prototype of the semi-classical approach to atomic and molecular structure.
Labelled Diagram Of An Atom Of Beryllium Labeled Diagram
Build an Atom - PhET Interactive Simulations

Bohr Diagram For Beryllium
Bohr model of sodium atom; beryllium atom Bohr model; In 1913, Danish physicist Neil Bohr proposed the Bohr atomic model based on Planck's quantum theory of radiation. This atomic model is the modification of Rutherford's atomic model (the nucleus is positively charged and is surrounded by electrons (negatively charged particles).
Periodic Table Beryllium Protons Neutrons Electrons Periodic Table
34 4.8K views 1 year ago In this video we'll look at the atomic structure and Bohr model for the Beryllium atom (Be). We'll use a Bohr diagram to visually represent where the electrons are.
Beryllium Bohr Model Diagram
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)
Beryllium Bohr Model Diagram
The calculation for the beryllium atom is carried out as shown below. Required student input is indicated by the highlighted regions. Enter nuclear charge: Z := 4 Kinetic energy: T1 ( R1 ) 1 := 2 ⋅ R12 1 T2 ( R1 ) := 8 ⋅ R12 Electron‐nucleus potential energy: − Z VN1 ( R1 ) := R1 − Z VN2 ( R1 ) := 4 ⋅ R1

Beryllium Bohr Model
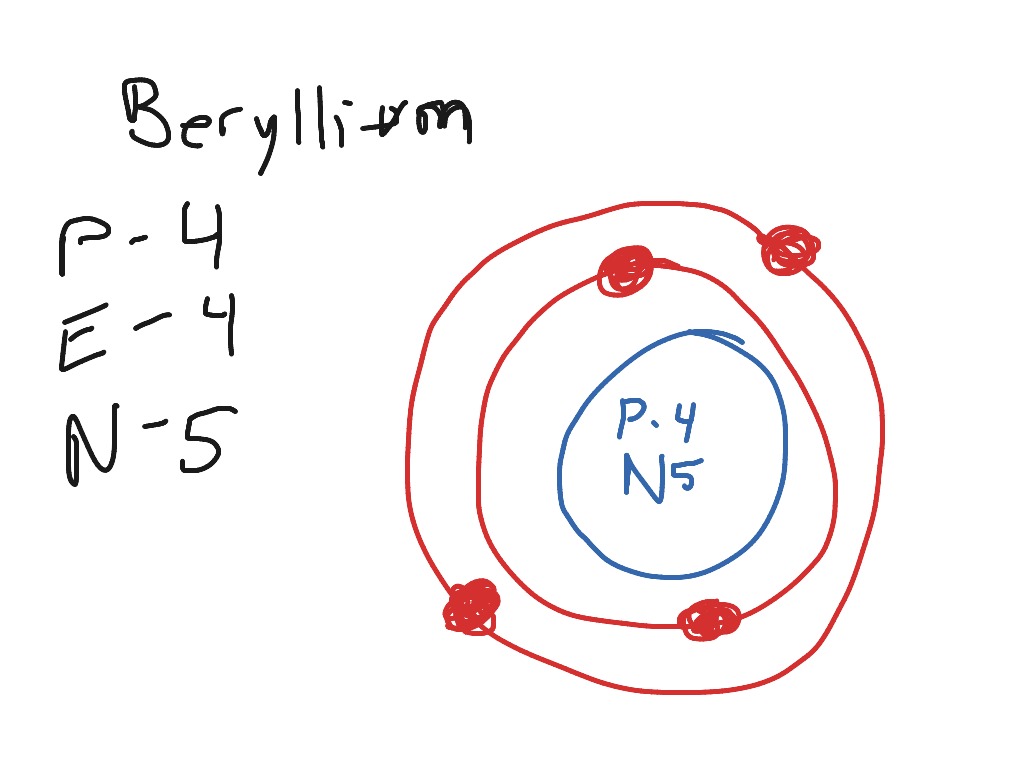
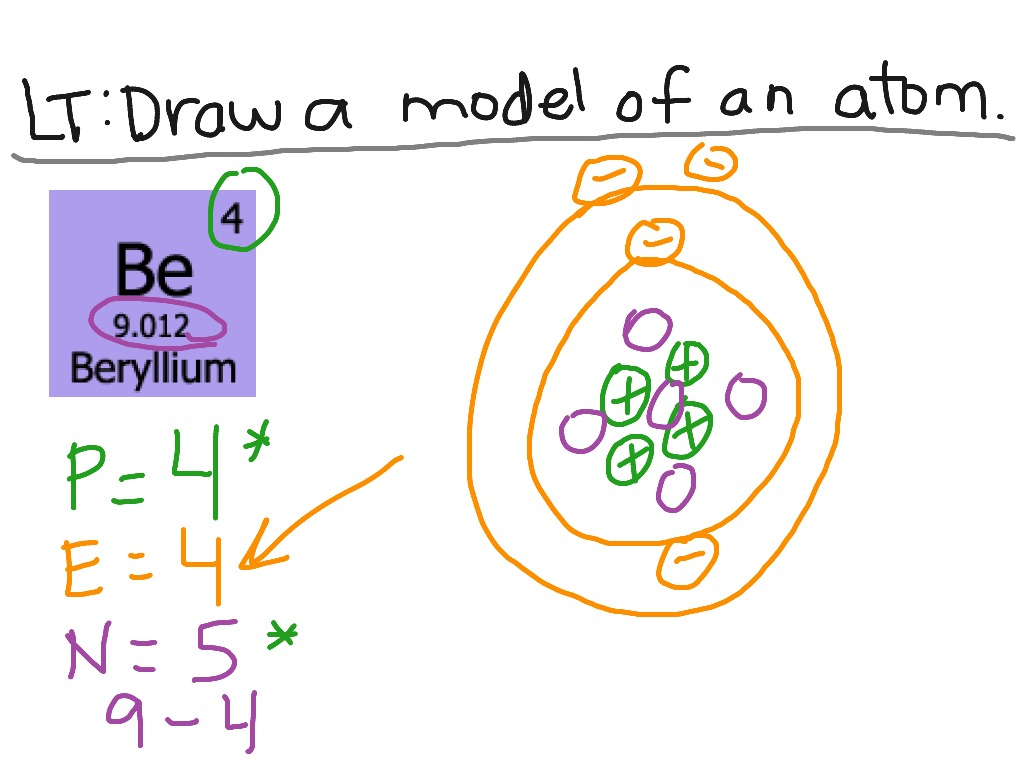
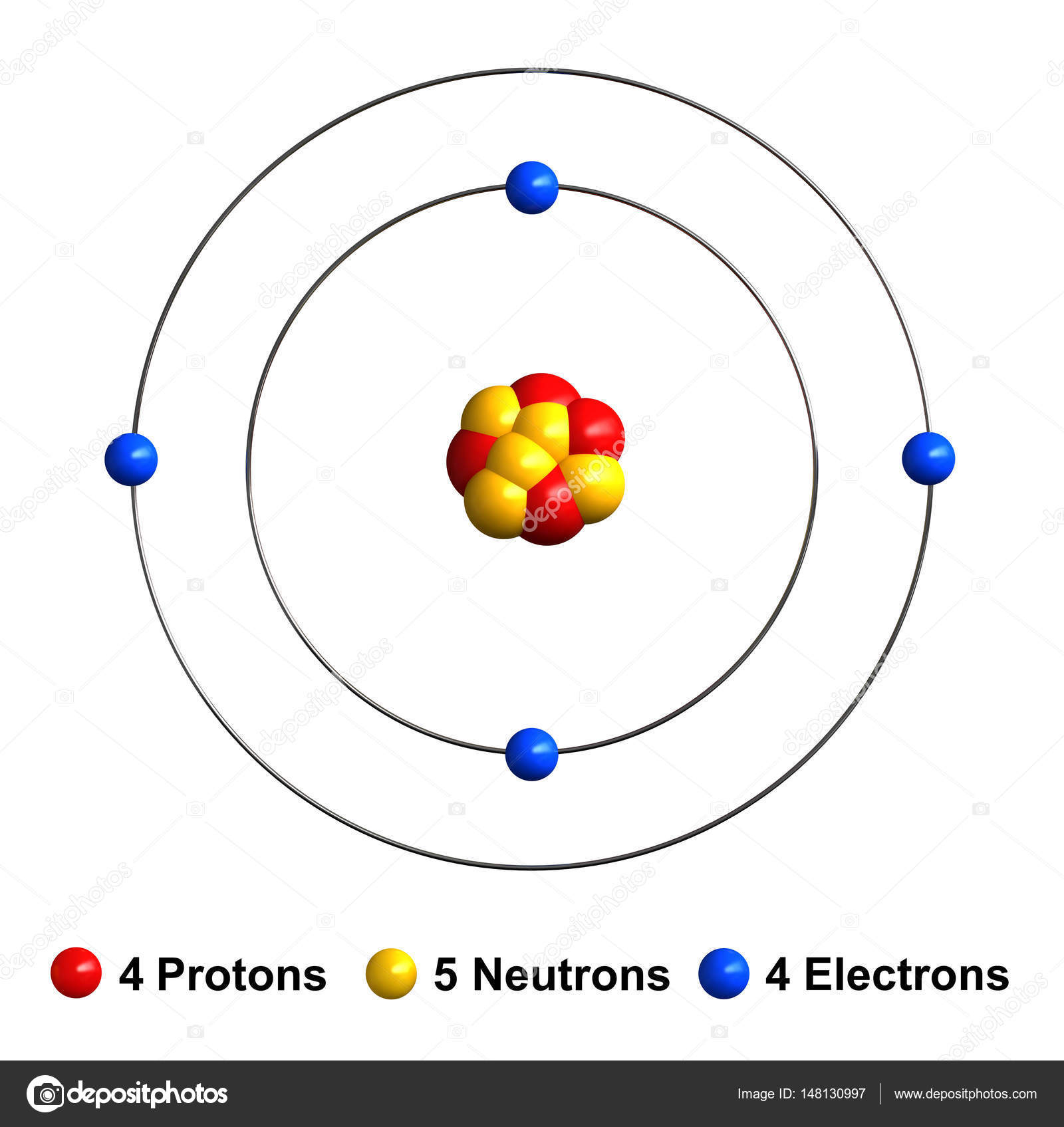
Steps Write protons, neutrons, and electrons of beryllium atom Beryllium has 4 protons, 5 neutrons, and 4 electrons. Learn how to find: Beryllium protons neutrons electrons Draw nucleus of beryllium atom The nucleus of a beryllium atom contains 4 protons and 5 neutrons. So draw the nucleus of beryllium atom as follows: Beryllium nucleus
Beryllium Bohr Diagram exatin.info
6.2 The Bohr Model; 6.3 Development of Quantum Theory; 6.4 Electronic Structure of Atoms (Electron Configurations) 6.5 Periodic Variations in Element Properties;. the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in.
Modelo Bohr de Átomo de Berilio con protones, neutrones y electrones
Atomic & Molecular Structure How to Make a 3-D Bohr Model ••• Updated April 24, 2017 By Tricia Lobo In your introductory chemistry classes you will have to become familiar with a number of the early models of atoms, which represent scientists' early concepts of the structure of atoms.

Beryllium Bohr Diagram Transborder Media
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.
Beryllium Element With Reaction, Properties and Uses Periodic Table
Ai = hν = hc λ A i = h ν = h c λ. Unknown wavelength λ λ can be determined from the Rydberg formula: 1 λ =R∞Z2( 1 n21 − 1 n22) 1 λ = R ∞ Z 2 ( 1 n 1 2 − 1 n 2 2) so that final equation for ionization energy looks like this: Ei = hc e R∞Z2( 1 n21 − 1 n22) E i = h c e R ∞ Z 2 ( 1 n 1 2 − 1 n 2 2)
Beryllium model Stock Vector Images Alamy
The model shown in the figure below is for the beryllium atom or any four‐electron ion. Note that the occupancy of the inner orbit is restricted to two electrons (Pauli Principle) and that the orbit radii are constrained by the hydrogen atom result (R 2 = 4R 1 ) leaving only one variational parameter, the radius of the n = 1 orbit.

Bohr Diagram For Beryllium General Wiring Diagram
The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. The Mass number is 9 which means Beryllium needs 5 neutrons in the nucleus. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons.
Beryllium Bohr Diagram exatin.info
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.