
ADL Alpha Line Plus Câbles stéréo RCA
In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron.The transitions are named sequentially by Greek letters: from n = 2 to n = 1 is called Lyman-alpha, 3 to 1 is Lyman-beta.

Tonalizante Alpha Line Essenziale 8.0 Louro Claro Ikesaki Cosméticos
The red H-alpha spectral line of the Balmer series of atomic hydrogen, which is the transition from the shell n = 3 to the shell n = 2, is one of the conspicuous colours of the universe.

What is the Alpha Line? YouTube
In fact, Bohr predicts that, in a star or in a vacuum tube containing hydrogen and helium and under proper conditions of excitation, the resulting spectrum will appear as in figure 4. The heavy unbroken lines are the first five members of the Balmer series. The dotted lines are the first ten members of the complete Pickering series, even values.

New Alpha line Focal
Buy it with. This item: Alphaline Studio Over-the-Ear Headphones | MoonPoint. $5999. +. Case Star Black Color Hard Shell Large Carrying Headphones Case/Headset Travel Bag for Sony MDR-ZX100 ZX110 ZX300 ZX310 ZX600 MDR-10RBT. $798. +. Apple Lightning to 3.5 mm Headphone Jack Adapter. $799.

D630 Tactic AlphaLine Wheels
7.3: The Hydrogen Spectrum. In 1885, J. J. Balmer, a lecturer in a ladies' college in Switzerland, devised a simple formula relating the wavelengths of the lines in the visible region of the atomic hydrogen spectrum to the natural numbers, and these lines have since been referred to as the Balmer series and have been denoted by H α α, H β β.

ALPHA LINE Cockpit Clean & Care Carpolish
Solution: We can use the Rydberg equation (Equation 1.5.2) to calculate the wavelength: 1 λ = RH( 1 n2 1 − 1 n2 2) A For the Lyman series, n1 = 1. 1 λ = RH( 1 n2 1 − 1 n2 2) = 1.097 × 107m − 1(1 1 − 1 4) = 8.228 × 106 m − 1. Spectroscopists often talk about energy and frequency as equivalent.

Conheça a Alpha Line Cosméticos Blog Vintage Pri Moda, beleza
H-alpha (Hα) is a deep-red visible spectral line of the hydrogen atom with a wavelength of 656.28 nm in air and 656.46 nm in vacuum. It is the first spectral line in the Balmer series and is emitted when an electron falls from a hydrogen atom's third- to second-lowest energy level. H-alpha has applications in astronomy where its emission can be observed from emission nebulae and from features.

Alpha Line Plus Ajasom
Shows two depth readings simultaneously from both transducers installed. Rudder (River) Turn (River) Shows the rudder angle (with needle down) is if required. Open the catalog to page 5. Tech Specs AlphaLine MFS-H 3803.0226 (grey), 3803.0228 (black) Weight 0.75 kg (1.65 lbs) 5-inch touch display 800-by-480-pixel resolution Horizontal.

Alpha Phi Alpha Line Jacket ubicaciondepersonas.cdmx.gob.mx
The Line Spectrum When light from a hot filament is dis persed according to wavelength by a prism or a diffraction grating. the result is a continuous fan of colors. but the spectrum from a pure. rarefied gas of atoms or molecules consists of discrete lines.

Alpha Line AD on Behance
The red line at the right is the \(H_{\alpha}\) line and the two leftmost lines are considered to be ultraviolet as they have wavelengths less than 400 nm. from Wikipedia. Balmer decided that the most likely atom to show simple spectral patterns was the lightest atom, hydrogen. Angstrom had measured the four visible spectral lines to have.

Alpha Line for LAcoustics Excellent Line
The Lyman-alpha line, typically denoted by Ly-α, is a spectral line of hydrogen (or, more generally, of any one-electron atom) in the Lyman series. It is emitted when the atomic electron transitions from an n = 2 orbital to the ground state ( n = 1), where n is the principal quantum number.

Máscara Alpha Line Liso Extraordinário 350g Top Profissional
The spectral series of hydrogen, on a logarithmic scale. The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom.

Tonalizante Alpha Line Extreme Colors Blue 100g Ikesaki Cosméticos
The K-alpha line in copper is frequently used as the primary source of X-ray radiation in lab-based X-ray diffraction spectrometry (XRD) instruments. K-beta K-beta emissions, similar to K-alpha emissions, result when an electron transitions to the innermost "K" shell (principal quantum number 1) from a 3p orbital of the third or "M" shell (with.

Calculated polarization difference profile of the Paschenalpha line of
Eminence Alpha 4-8 4" Full-Range Pair 8 OhmPerfectly suited for line arrays, car doors and side panels, and other tight fitting applications, Eminence's Alpha 4-8 is a very versatile 110 watt maximum driver that can be used as a full range, midbass, or midrange. They can be stacked by themselves in a column for vocal applications, or in conjunction with a tweeter and a sub for compact, high.
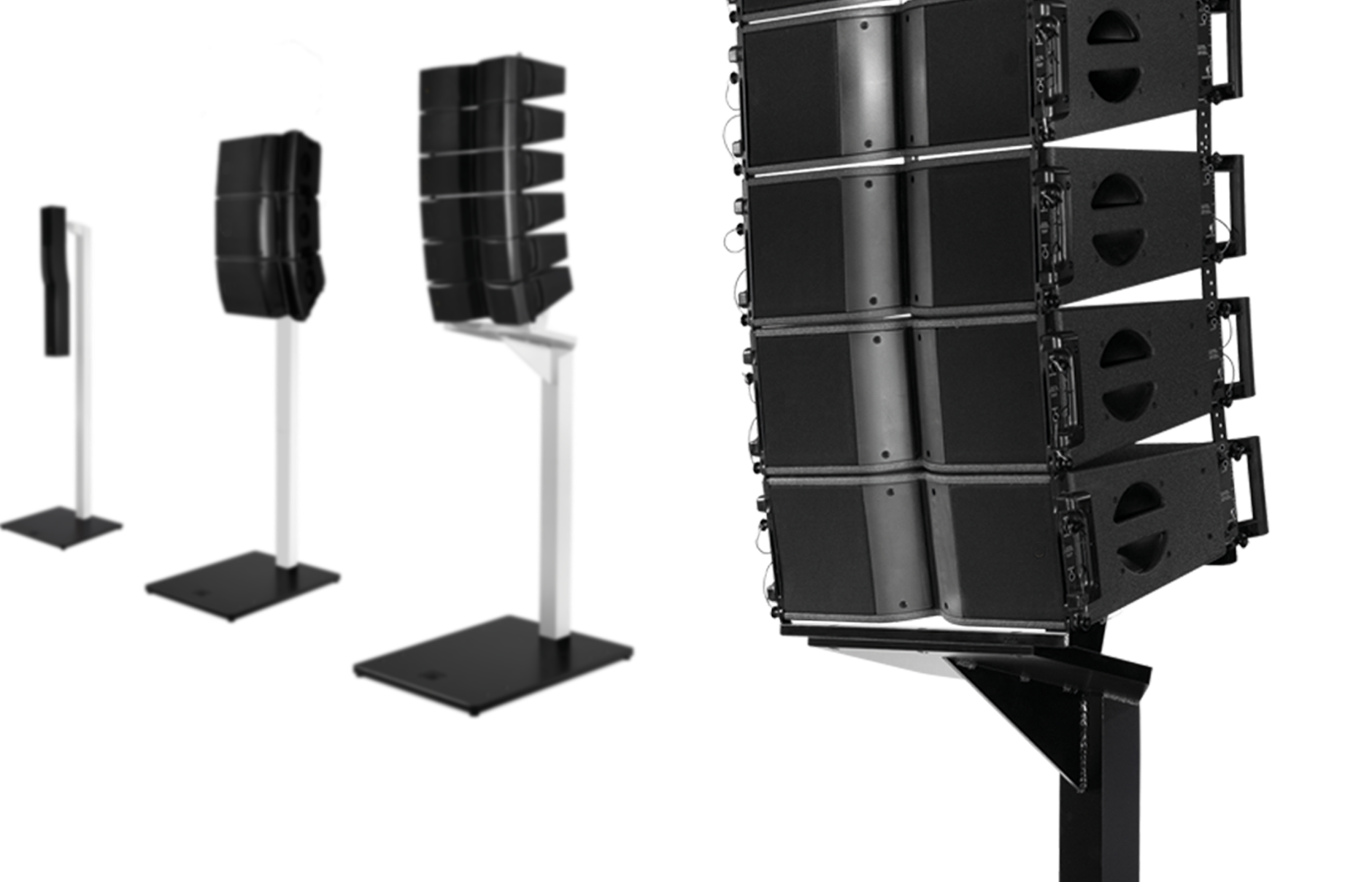
Alpha Line for LAcoustics Excellent Line
Substitute the appropriate values into Equation \ref {6.3.2} (the Rydberg equation) and solve for \ (\lambda\). Use Figure 2.2.1 to locate the region of the electromagnetic spectrum corresponding to the calculated wavelength. Solution: We can use the Rydberg equation to calculate the wavelength:

ALPHA LINE Autoduft & Geruchskiller Car Care King
Summary. This update addresses a security vulnerability that could allow attackers to bypass BitLocker encryption by using Windows Recovery Environment (WinRE).