
Catatan BCS Biopharmaceutical classification system CATATAN BIOPHARMACEUTICAL CLASSIFICATION
BCS classification system is an important tool for generic drug development. It's give a comparative evidence between test product and RLD (reference listed drug). Without BCS classification it's so tough to design a generic drug development. Because the solubility and permeability of API highly impact on BE study.
Bcs classification system
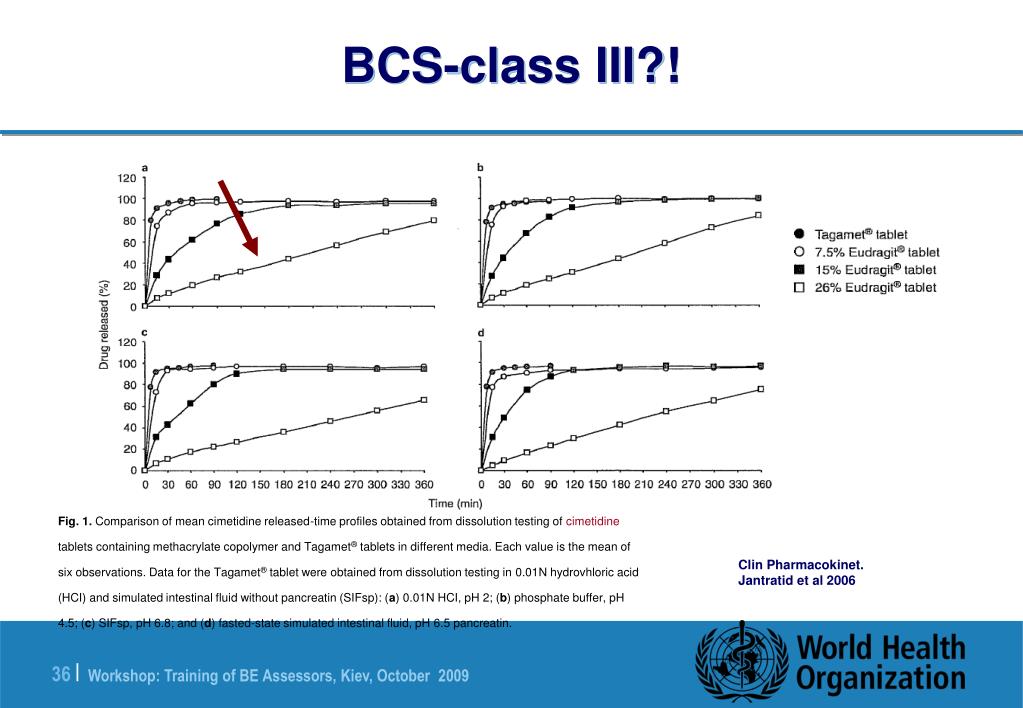
However, important concerns for BCS class III drugs are the effect of excipients on GI transit and permeability (both passive and carrier-mediated uptake and/or efflux transport). 109 Further support for biowaiver of BCS class III drugs is available from Vogelpoel et al., who showed recently that atenolol is a candidate for biowaiver, provided.
Bcs classification system
The BCS Class III compounds are hydrophilic molecules (high aqueous solubility) with low permeability across the biological membranes. While these compounds are pharmacologically effective, poor.

SOLUTION Sistem klasifikasi biofarmasetika bcs Studypool
Learning Objectives. To discuss essential elements of Biopharmaceutics Classification System (BCS) III-based biowaiver as an alternative bioequivalence (BE) approach. To share research results on.

Biopharmaceutics Classification System Chart
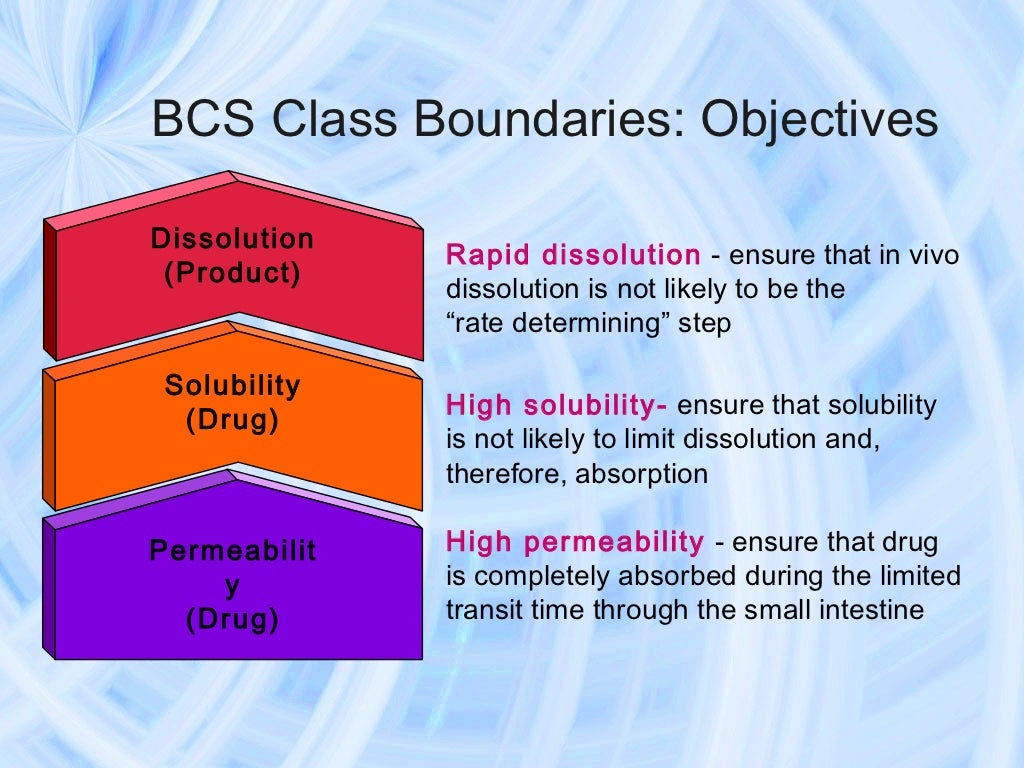
BCS mengklasifikasikan obat menjadi 4 kelompok sesuai dengan tingkat permeabilitas usus dan kelarutan airnya yaitu: kelas 1 (permeabilitas tinggi, kelarutan tinggi), kelas 2 (permeabilitas tinggi, kelarutan rendah), kelas 3 (permeabilitas rendah, kelarutan tinggi), dan kelas 4 (permeabilitas rendah, kelarutan rendah) (Amidon et al., 1995.

Significance Of Bcs Classification
INTRODUCTION. The United States Pharmacopeial Convention (USP) authorized the creation of an advisory panel to investigate the possibility of applying the principles of the Biopharmaceutics Classification System (BCS) to veterinary drugs—specifically, solid oral formulations administered to dogs ().Developed for human pharmaceutical compounds (2-6), the BCS is an important tool that.

Bcs Kelas IV PDF
THE BCS AND ITS USE IN DRUG DEVELOPMENT. The BCS characterizes drugs into four classes according to their US FDA solubility and permeability as depicted in Figure 1.In 2000, the US FDA promulgated the BCS system as a science-based approach to allow waiver of in vivo bioavailability and bioequivalence testing of immediate-release solid oral dosage forms for Class 1 high solubility, high.

SOLUTION Sistem klasifikasi biofarmasetika bcs Studypool
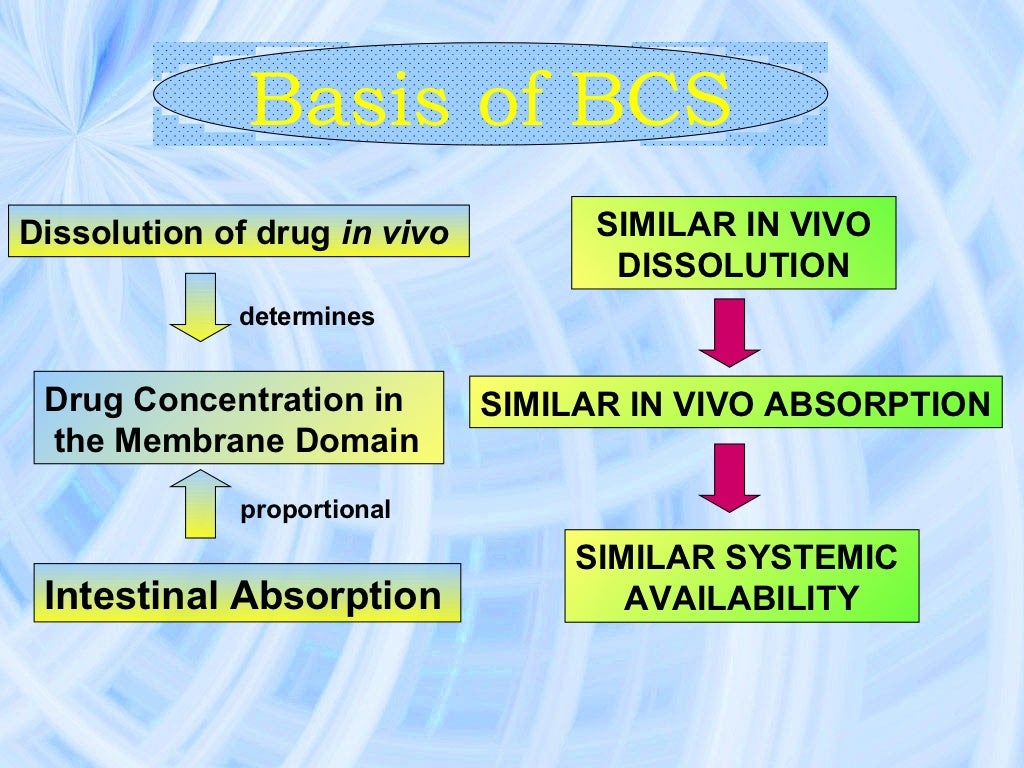
Similarity has to be assessed over the entire physiological pH range (e.g. at pH 1.3, 4.6 and 6.8) by comparing the dissolution curves by means of the f 2 equation (FDA, 1997). 1.2.. The BCS was primarily developed for a better understanding of the relationship of drug release (in vivo) from the product and the absorption process. In this.
PPT Training Training of BE Assessors Kiev, October 2009 BCSbased Biowaivers
3. Klasifikasi BCS. BCS (Biopharmaceutical Classification System) atau sistem klasifikasi biofarmasetika diklasifikasikan. menjadi empat kelas, diantaranya adalah : 1. Kelas I (Permeabilitas tinggi, Kelarutan tinggi) Misalnya Metoprolol, Diltiazem, Verapamil, Propranolol. Obat kelas I menunjukkan penyerapan yang. tinggi dan disolusi yang tinggi.

BCS kelas 1
Zinc Sulfate (BCS Class - III) Zolpidem tartrate (BCS Class - I) Zuranolone (BCS Class - II/IV) << Previous Page (A-J) Related Topics. BCS Class 1 Drugs List; BCS Class 2 Drugs List; BCS Class 3 Drugs List; BCS Class 4 Drugs List; Recommended also. Pharma Books Online Review; Drug Excipients Database;

The Biopharmaceutics Classification System (BCS) as defined by Amidon... Download Scientific
Introduction. The drug absorption rate in gastrointestinal (GI) tract is impacted by plenty of factors, like physicochemical nature, size and molecular weight of the compounds, metabolic, physiological functions, structure and surface of the gut cells etc. 1,2 Notwithstanding this complexity, the Bio pharmaceutics Classification System (BCS) developed by Amidon et al. 3 and Lipinski et al.

Bcs classification system
Despite the high Log P value for furosemide, it was indeed confirmed that furosemide is a BCS class IV drug, based on both the solubility data (Table 1) and the intestinal permeability (Figure 3). Suitable formulation is the main approach to create an efficacious drug product for the administration of BCS class IV drugs . Absorption windows in.

(DOC) Bahan BCS Boen Juan Academia.edu
The BCS categorizes drug substances into one of four biopharmaceutic classes, as follows: Class I: high solubility, high permeability. Class II: low solubility, high permeability. Class III: high solubility, low permeability. Class IV: low solubility, low permeability. This classification scheme allows defines standards conditions for in vitro.

Body Condition Score (BCS) Untuk Efisiensi Kinerja Reproduksi Sapi PB ISPI
increase in solubility of 362.71± 3.6 µgm/mL as compared to the pure Spl alone at 34.48 ± 1.69 µgm/mL. Drug content in all the SDs was uniform with the lowest being 93.14 ±. increase the solubility of the BCS Class II model drug spironolactone when formulated in a ternary solid dispersion along with the β-CD. Dedicated to Mom and Dad :
Bcs Class 2 Drug List Pdf ographypotent
A drug product is eligible for a BCS-based biowaiver provid that the drug substance(s) satisfy the ed criteria regarding solubility and permeability (BCS Class I and III), the drug product is an immediate-release oral dosage form with systemic action, and the drug product is dosage the same form and strength as the reference product.
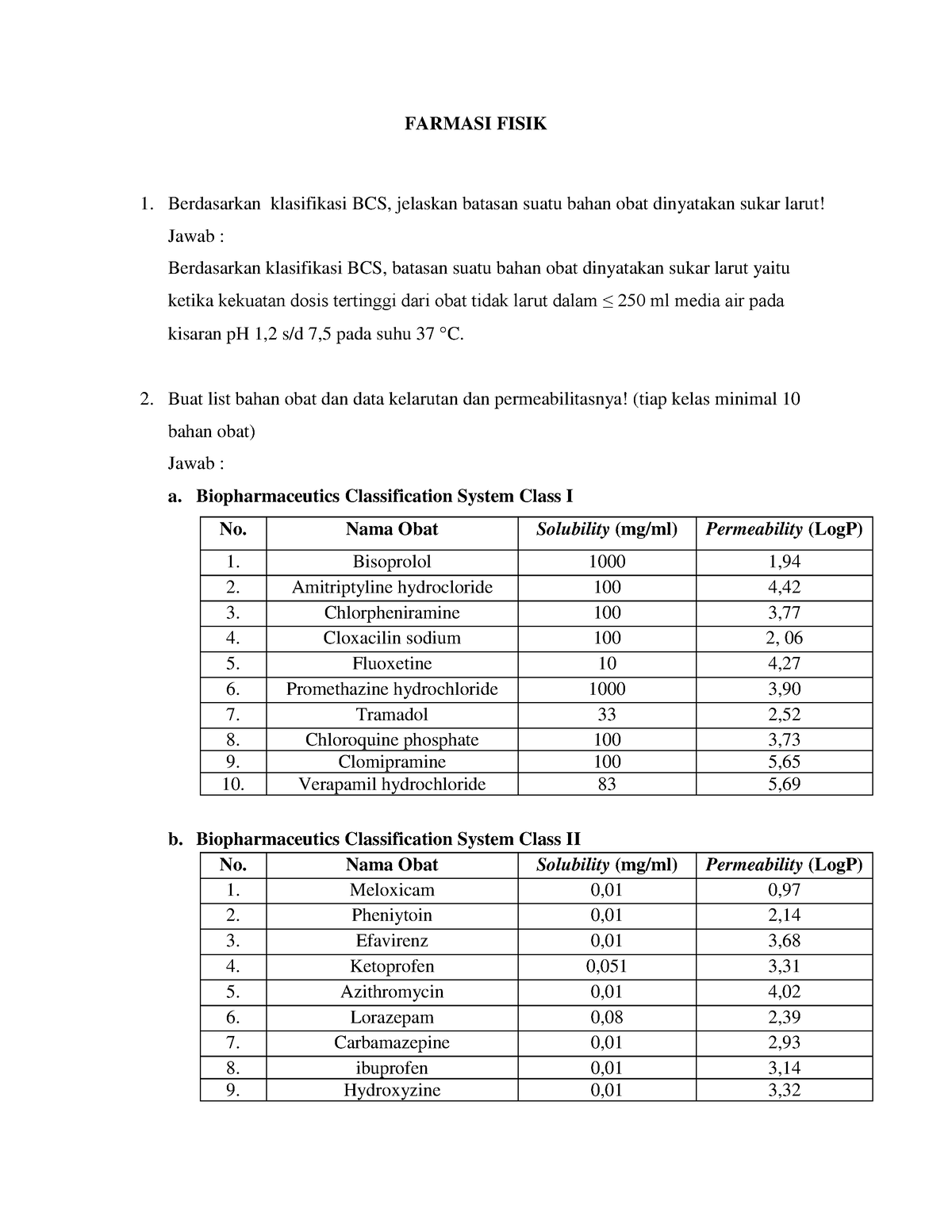
Farmasi Fisik BCS FARMASI FISIK Berdasarkan klasifikasi BCS, jelaskan batasan suatu bahan obat
BCS kelas II, III dan IV dikembangkan oleh peneliti dengan menggunakan formulasi ko-amorf dalam memperbaiki sifat kelarutan dan permeabilitasnya yang terdapat pada Tabel 1. Obat pada Tabel 1